Figure 2. Colocalization of Tid1 and AChRs in C2C12 Myotubes and on the Postsynaptic Membrane of the NMJ.
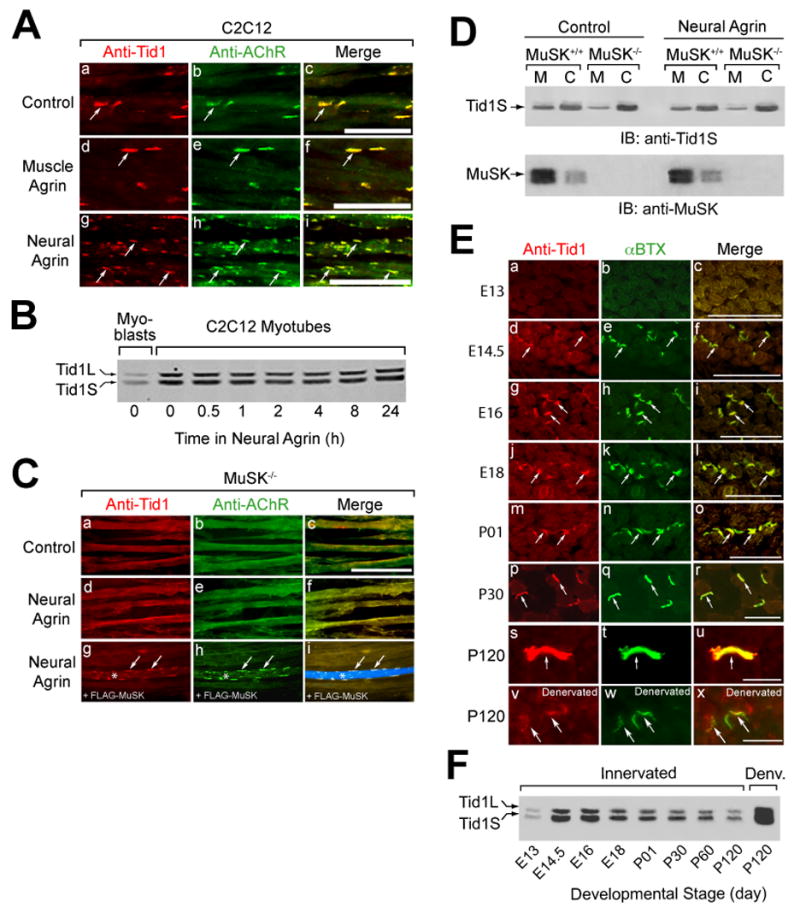
(A) Tid1 is co-clustered with AChRs (arrows) on the surface of C2C12 myotubes. C2C12 myotubes were incubated with control medium, muscle agrin (CAg12,0,0, an alternatively-spliced isoform with little AChR clustering activity), or neural agrin (CAg12,4,8) for 4 h, fixed, and double-stained with a pan-Tid1 rabbit polyclonal antibody (anti-Tid1) and mAb 35, a rat monoclonal antibody against the AChR. Cy3-conjugated goat anti-rabbit and Cy2-conjugated goat anti-rat IgGs were used as secondary antibodies. Scale bar = 25 μm in (A), (C), and (E). (B) The effects of differentiation and agrin on the expression of Tid1 in C12C12 cells. Myoblasts (lane 1) were maintained in growth medium; myotubes were grown in fusion medium for 48 hrs. Myotubes (lanes 2–8) were treated with agrin for the indicated time, lysed with extraction buffer, and immunoblotted with mAb RS-13. (C) Agrin does not cluster Tid1 or AChRs without MuSK. Myotubes derived from MuSK−/− mice were treated for 4 h with neural agrin and stained as in (A) for Tid1 and with Alexa Fluor 488-conjugated α-bungarotoxin (αBTX) to label AChRs (d–f). In a rescue experiment (g-i), MuSK−/− cells were transfected with a plasmid cDNA encoding FLAG-MuSK, treated with neural agrin, and stained with the anti-FLAG M2 antibody followed by an Alexa Fluor 633-conjugated rabbit anti-mouse secondary antibody. A FLAG-MuSK-positive myotube is clearly visible in i (*, arrows). (D) More Tid1S is found in the cytosolic versus the membrane fraction of MuSK-deficient muscle cells, as compared to wildtype myotubes. Myotubes derived from the skeletal muscles of MuSK+/+ or MuSK−/− mice were treated with or without neural agrin, homogenized, and fractionated by ultracentrifugation. The amount of Tid1S protein in the membrane (M) and cytosolic (C) fractions was determined by immunoblotting with a rabbit polyclonal anti-Tid1S antibody (anti-Tid1S). (E) Tid1 is concentrated at motor endplates (arrows) during development. Cross-sections of mouse hindlimb muscles at different ages were stained as in (C). 7.5 weeks after denervation, denervated (v-x) and contralateral innervated (s-u) soleus muscles were dissected, cryosectioned, fixed, and stained. (F) The expression of Tid1 in mouse skeletal muscle during development and following denervation. After homogenization of mouse hindlimb muscles, cleared lysates were immunoblotted with mAb RS-13. An equal amount of total protein was loaded into each of the lanes.
