Figure 7. Tid1 Acts Downstream of Dok-7.
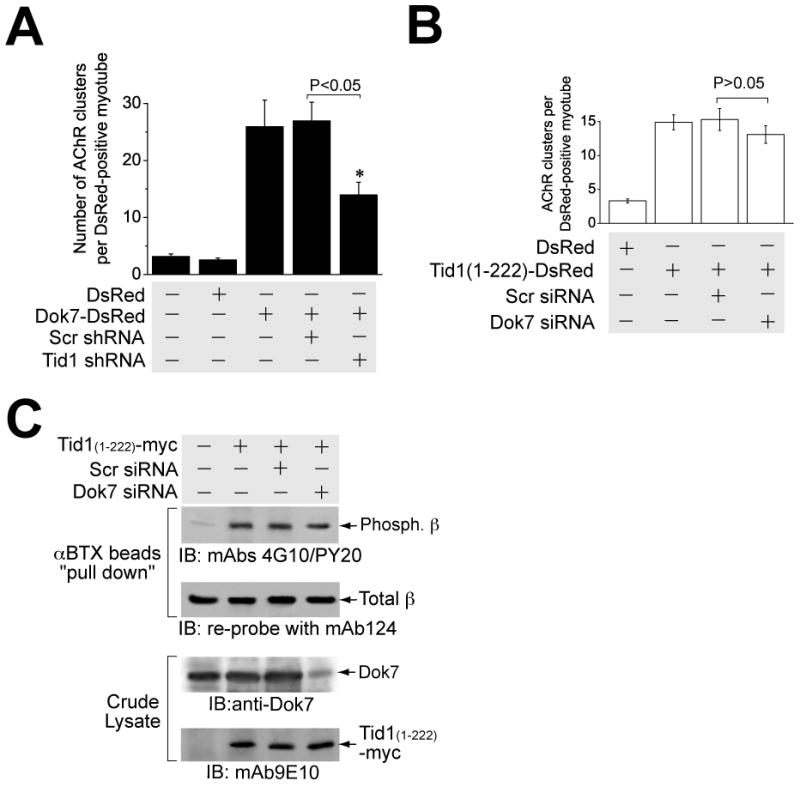
(A) Tid1 shRNA disrupts Dok-7-induced AChR clustering. C2C12 myoblasts were transfected with plasmids encoding DsRed (control), Dok-7-DsRed, or Dok-7-DsRed together with Scr or Tid1-specific shRNA, respectively. After differentiation, myotubes were fixed briefly and stained with Alexa Fluor 488-conjugated αBTX. The results represent mean ± SEM of four independent experiments. *, p < 0.05. n = 60 myotubes for each of the experimental groups. (B) Dok-7 siRNA has no significant effect on AChR clustering induced by the overexpression of Tid1(1–222). C2C12 myoblasts were transfected with plasmid cDNAs encoding DsRed (control), Tid1(1–222)-DsRed, or Tid1(1–222)-DsRed together with Scr siRNA or Dok-7 siRNA, respectively. After differentiation, myotubes were fixed and stained with Alexa Fluor 488-conjugated αBTX. The results represent mean ± SEM of four independent experiments. n = 80 myotubes for each of the experimental groups. (C) AChR β subunit phosphorylation induced by the N-terminal domain of Tid1 is not affected by Dok-7-targeted siRNA. C2C12 myoblasts were transfected with plasmid cDNAs encoding myc-tagged Tid1(1–222), along with either control (Scr) or Dok-7 siRNA, respectively. Myotubes were lysed with extraction buffer and assayed for β phosphorylation as described in Fig. 6B. Crude lysates were immunoblotted with rabbit anti-Dok-7 and mAb 9E10 antibodies.
