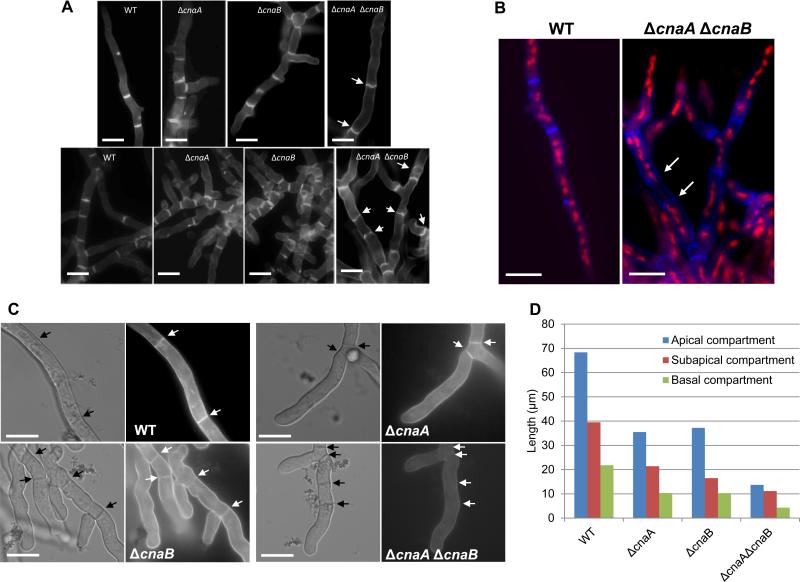
Figure 4.
(A). Calcofluor white staining of the wild-type and calcineurin mutant strains. A total of 1×104 conidia of the wild-type and the calcineurin mutant strains were inoculated onto coverslips immersed in GMM medium and grown for 24 h at 37°C. The coverslips were rinsed in sterile water, and inverted onto a 200 μl drop of calcofluor white staining solution for 5 minutes at room temperature. Coverslips were then rinsed twice for 10 minutes in sterile water and observed under fluorescent microscope. Scale bar, 10 μm. (B). For dual staining with calcofluor white and propidium iodide to observe septa and nuclei, first nuclear staining was performed by fixing the hyphae in 8% formaldehyde and staining with propidium iodide solution (12.5 μg/ml) in 50 mM PIPES (pH 6.7) for 5 min and then calcofluor white staining for 5 minutes at room temperature and observed under the fluorescence microscope. Scale bar, 10 μm. (C) Staining of cell wall β-glucan with aniline blue was performed on cultures grown on coverslips by inverting them onto a 200 μl drop of aniline blue (0.1 mg/ml) staining solution for 5 min at room temperature. Stained mycelia were directly observed by fluorescence microscopy. Note the absence of aniline blue staining of the septa in the ΔcnaA ΔcnaB strain. Arrows indicate septa. Scale bar, 10 μm. (D) Hyphal growth defect was quantified by measuring the apical, subapical and basal hyphal compartments in the single (ΔcnaA and ΔcnaB) and double (ΔcnaA ΔcnaB) mutants after growth for a period of 24 and 48 hours, respectively. Approximately 100 hyphal compartments were measured in each strain and the data depicts average lengths of the various compartments