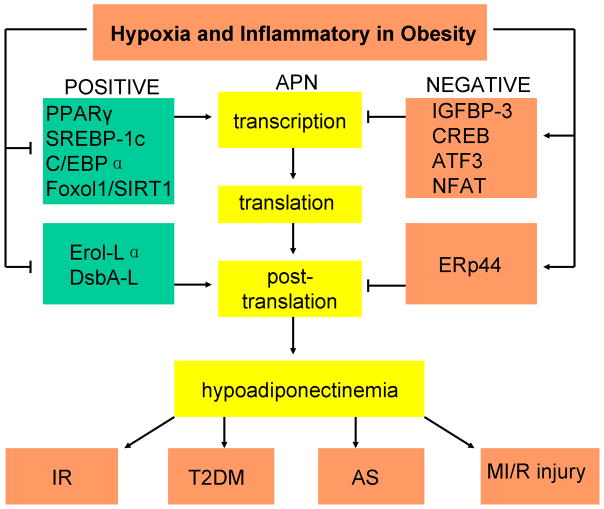
Figure 1. Relationships and mechanisms underlying obesity and hypoadiponectinemia, and the consequences of hypoadiponectinemia.
Many transcription factors positively and negatively regulate adiponectin (APN) gene expression. Positive transcription factors, such as PPARγ, SREBP-1c, C/EBPα, and Foxol1/SIRT1, increase APN gene transcription. Negative transcription factors, such as IGFBP-3, CREB, ATF3, and NFAT, suppress APN gene transcription. Several molecular chaperones regulate the post-translation of APN, including Ero1-Lα, DsbA-L and ERp44. ERp44 retains APN intracellularly, while Ero1-Lα and DsbA-L facilitate APN multimerisation and secretion. In obesity, increased fat mass results in adipose tissue hypoxia, increasing endoplasmic reticular stress. Obesity is a low-grade chronic inflammatory state. ER stress and increased pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-18 can inhibit the effect of positive transcription and post-translation factors, inducing production of inhibitory transcription factors, resulting in hypoadiponectinemia. Insulin resistance, type 2 diabetes, atherosclerosis formation, and increased myocardial ischaemia/reperfusion injury are all consequences of hypoadiponectinemia, and may all share a common etiological mechanism.