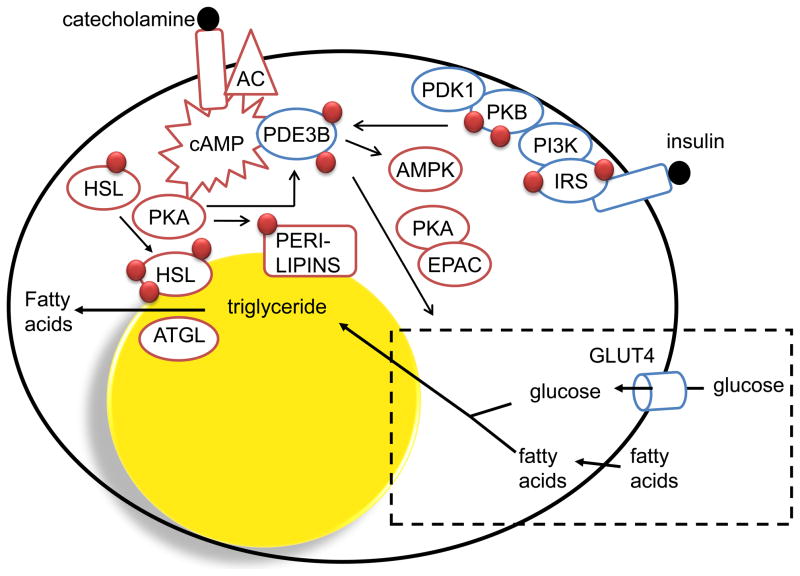
Figure 3. Role of PDE3B in hormone-mediated regulation of adipocyte functions.
Activation of PDE3B leads to increased hydrolysis of cAMP and thereby inhibition of catecholamine-induced lipolysis, a process which involves PKA-dependent phosphorylation of hormone-sensitive lipase (HSL) and perilipin. Insulin-mediated phosphorylation and activation of PDE3B involves tyrosine phosphorylation of insulin receptor substrates (IRS) catalyzed by the activated insulin receptor tyrosine kinase (IRTK), activation of PI3K and increased production of phosphatidyl inositol 3,4/3,4,5 phosphates. This is followed by the activation of PKB which is believed to be one important kinase that phosphorylates and activates PDE3B. Phosphorylation and activation of PDE3B by cAMP-increasing hormones are thought to be important in feedback-regulation of cAMP and cAMP-mediated responses. PDE3B is also important in insulin-induced regulation of glucose uptake and lipogenesis which involves PKA as well as Epac proteins. Finally, PDE3B as well as PDE4 seem to regulate cAMP pools that affect the activation/phosphorylation state of AMPK. ATGL (adipose triglyceride lipase).