Figure 3.
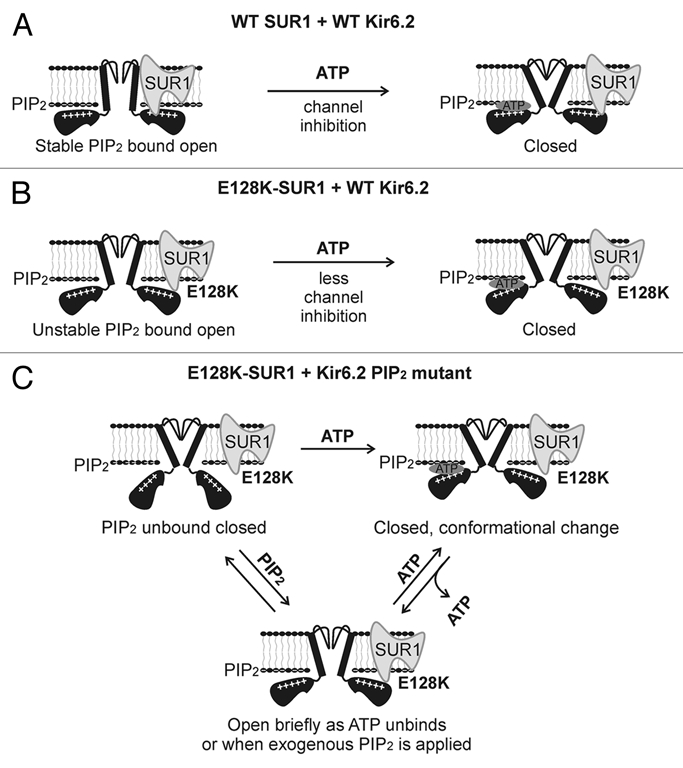
Proposed mechanism by which ATP stimulates the activity of channels formed by E128K-SUR1 and R176E-, R192E- or R206E-Kir6.2. (A) In WT channels SUR1 and Kir6.2 are functionally coupled. This leads to stable interactions between Kir6.2 and PIP2 and efficient ATP inhibition of channel activity. (B) In E128K-SUR1 + WT Kir6.2 channels, functional coupling between SUR1 and Kir6.2 is disrupted causing unstable Kir6.2-PIP2 interactions thereby reduced open probability. The uncoupling between SUR1 and Kir6.2 also renders the channel less sensitive to ATP inhibition. (C) In channels formed by E128K-SUR1 and Kir6.2 PIP2 mutants, most channels are unable to interact with PIP2 resulting in no channel activity in Kint/EDTA. ATP binding to Kir6.2 causes a conformational change that resets the channel from closed to a “ready to conduct” state such that when ATP unbinds, the channel opens briefly before relapsing into PIP2-unbound closed state or before ATP rebinds again to close the channel. High concentrations of exogenous PIP2 can also force the channel to open briefly. Note schemas do not represent quantitative kinetic or structural information.
