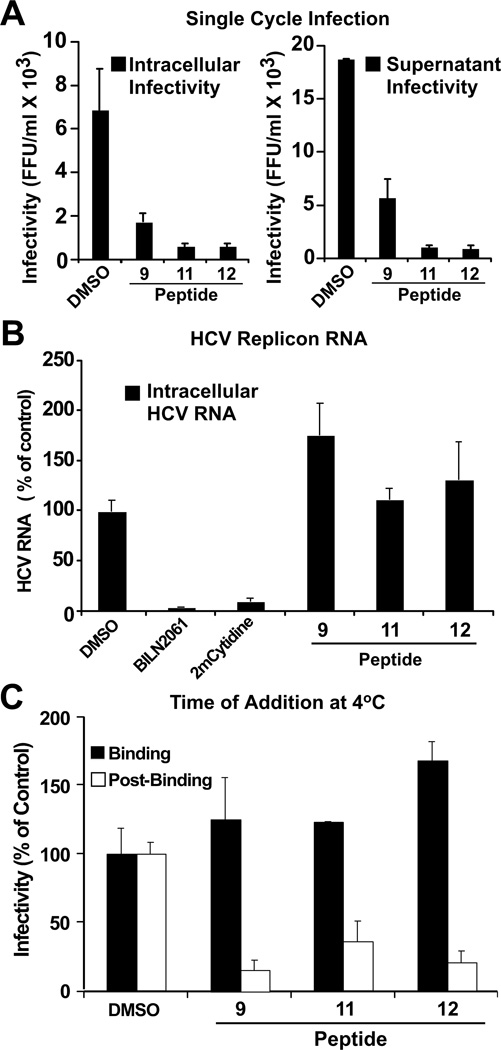
Figure 2. d,l-α-peptides inhibit infection in a post-binding step preceding viral RNA replication.
(A) Single cycle infection in the presence of antiviral peptides. Huh-7 cells were inoculated at multiplicity of 5 (m.o.i 5) with HCV for 5 h at 37 °C in the presence of vehicle (DMSO) or the designated peptide (20 µM). After the inoculation period, the cells were washed once with PBS and further incubated in complete medium without peptides. Infectivity was determined in total cell extracts and supernatants 24 h post-inoculation. Intracellular infectivity (FFU/ml) (Left). Extracellular infectivity (FFU/ml) (Right). Data are shown as average and mean error of duplicate infections. *Peptide 4 not shown, tested under same conditions displayed inconsistent results.
(B) d,l-α-peptides do not display anti-HCV activity in the replicon model. Huh-7 cells harboring a full-length JFH-1 replicon were treated with cyclic peptide (20 µM), the protease inhibitor BILN2061 (1 µM), or the polymerase inhibitor 2’-C-methyl-cytidine (1 µM) for 48 hours at 37 °C. Intracellular HCV RNA levels were determined by RT-qPCR and normalized with GAPDH mRNA levels. Results are shown as average and mean error of triplicate experiments.
(C) d,l-α-peptides do not interfere with infectious particle binding but target a post-binding step of the viral lifecycle. Huh-7 cells were inoculated with increasing amounts of infectious HCV for 1 h at 4 °C in the presence of a constant amount of peptide (20 µM). Susceptibility of binding or post-binding events to inhibition was studied by adding the peptide during inoculation or after virus particle binding to target cells (see Materials and Methods). Infection efficiency was calculated by determining the infectivity titer in each condition and comparing it with that obtained in the presence of vehicle (DMSO).