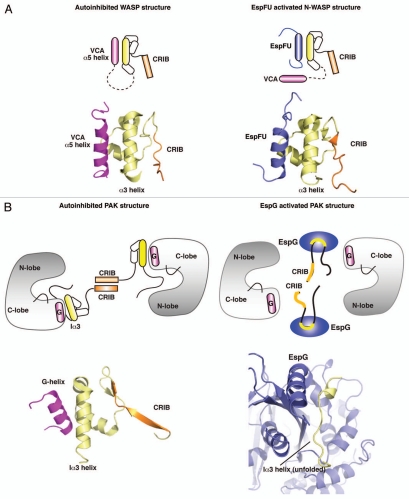
Figure 1.
Structural principles for the activation of WASP and PAK proteins by bacterial effectors. (A) Left part: cartoon and structural models of autoinhibited WASP, showing the interaction of VCA (magenta) with GBD (yellow). (PDB ID: 1EJ5) Right part: cartoon and structural models showing the binding of EspFu helix (blue) to GBD, leading to the release of VCA and activation of WASP signaling. (PDB ID: 2K42). (B) Left part: cartoon and structural models of autoinhibited PAK1 homodimer, and close up view of the autoinhibitory domain organization (yellow) and G-helix of the Kinase Domain. (PDB ID: 1F3M) Right part: Binding of EspG (blue) to PAK unfolds Ia3-helix (yellow) and leads to the disruption of inhibitory contacts, activating the kinase. (PDB ID: 3PCS).