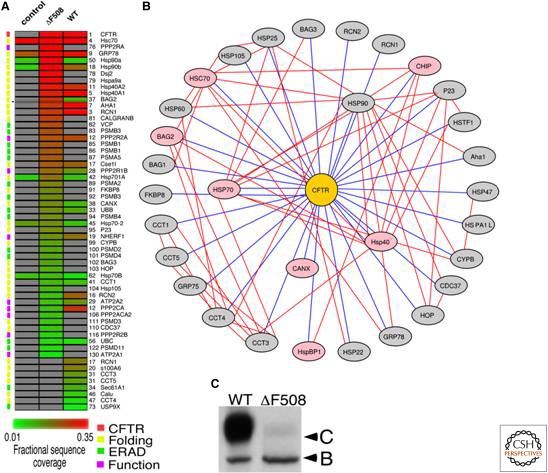
Figure 9.
Characterization of proteostasis network components used by the client protein cftr using immunoisolation followed by mass spectrometry. The cystic fibrosis transmembrane conductance regulator (CFTR) interactome (panels A and B) was characterized by immunoisolating both wild type and mutant (ΔF508) CFTR followed by characterization of the interacting proteins by MudPIT mass spectrometry. A ΔF508 CFTR folding intermediate in the cytosol appears to be sequestered by the Hsp90 chaperone–Aha1 cochaperone complex leading to endoplasmic reticulum-associated degradation and poor secretion (Panel C: note lack of C band reflecting CFTR on plasma membrane). Reducing the concentration of Aha1 enhances the folding of ΔF508 CFTR by altering the proteostasis network in such a fashion that it can now more efficiently fold ΔF508 CFTR. Figure kindly provided by William E. Balch.