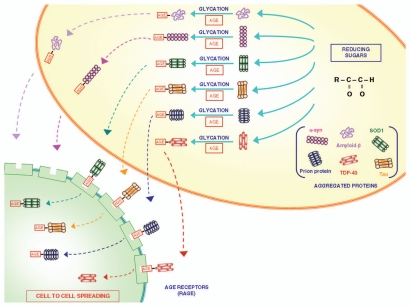
Figure 2.
The role of glycation in modifying proteins structure and function. Glycation is a post-translational, non-enzymatic reaction between reducing sugars and amino groups, resulting in the formation of AGE. The major glycation agents include methylglyoxal, glyoxal and 3-deoxyglucosone. Misfolded proteins represent an important target for glycation. A variety of proteins involved in neurodegenerative disorders (such as Aβ, SOD-1, α-synuclein, TDP-43, tau protein and prion protein) undergo glycation, responsible for an increased stability by the formation of crosslinks that stabilize protein aggregates. These AGE are expressed on the plasma membrane and may diffuse extracellularly to interact with neighbouring cells to spread according to a prion-like mechanism.34 In fact, glycated proteins would interact with RAGE, thus allowing a “cell to cell spreading” by their internalization. This process, which is well demonstrated within CNS, remains at hypothetical level within the gut, where in any case the prion protein is shown to spread from cell to cell.