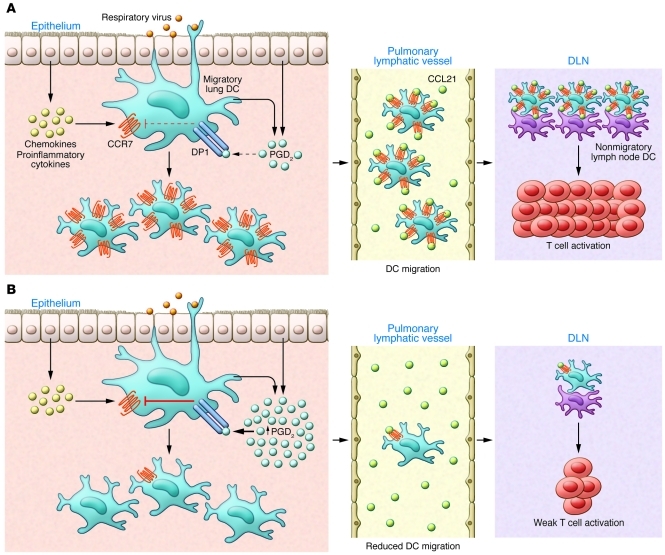
Figure 1. Impact of aging on the induction of adaptive antiviral immunity.
(A) Recognition of the invading respiratory virus by innate immune receptors expressed by epithelial cells and innate immune cells, including mast cells and DCs, in the lungs leads to the production of proinflammatory cytokines (i.e., IL-1β, IL-18, and type I IFNs), chemokines (i.e., MCP-1 and CCL2), and AA-derived metabolites (i.e., PGD2, PGE2, and LTC4). Of note, basal levels of prostanoids, in particular PGD2, are increased in the lungs of aged mice. The lipid and soluble mediators in turn enhance the expression of receptors such as DP1 on DCs in the lungs. Typically in young individuals, the proinflammatory milieu in the lungs created in response to viral infection recruits and activates adjacent DCs. Activated lung DCs undergo a maturation process that includes upregulation of costimulatory ligands, antigen-presenting complexes, and, importantly, chemokine receptors (such as CCR7 and sphingosine-1-phosphate receptors [S1P1–5]). The elevated levels of chemokine receptors facilitate emigration of antigen-bearing lung DCs to the local secondary lymphoid organs draining the infected lung (DLNs), in which they participate in initiating adaptive immune response to the respiratory virus. (B) However, in the lungs of the aged, the elevated basal levels of PGD2 in the lung tissue suppress CCR7 expression on lung DCs with migratory capacity. This reduced CCR7 expression by the lung DCs impairs DC migration from the infected lung to the DLN and results in fewer viral antigen-bearing lung DCs available to activate naive virus-specific T cells.