Figure 2.
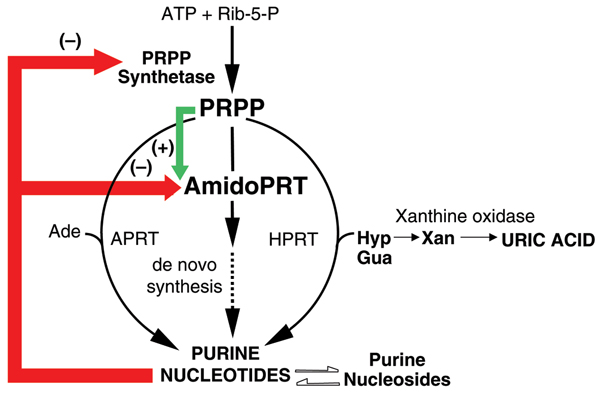
Xanthine oxidase in the context of purine metabolism. Schematic representation of human purine metabolic pathways, culminating in the production of uric acid. Purine nucleotides are synthesized by alternative pathways, each requiring the key regulatory intermediate 5-phosphoribosyl 1-pyrophosphate (PRPP), which is synthesized from ATP and ribose-5-P in a reaction catalyzed by PRPP synthetase. The pathway of purine synthesis de novo involves a sequence of 10 reactions by means of which a purine ring is synthesized on a ribose-phosphate backbone donated by PRPP. The first reaction in the pathway is the rate-limiting step and is catalyzed by the enzyme amidophopshoribosyltransferase (AmidoPRT). The subsequent 9 reactions in the de novo pathway are represented by the dashed arrow. The alternative pathways of purine nucleotide synthesis are single step processes by which preformed purine bases (adenine, Ade; hypoxanthine (Hyp); guanine, Gua) are salvaged in reactions catalyzed by the phosphoribosyltransferase (PRT) enzymes adeninePRT (APRT) and hypoxanthine-guanine (HPRT), respectively. Regulation of nucleotide synthesis is effected mainly at the AmidoPRT step, by means of antagonistic allosteric regulation of the activity of AmidoPRT by inhibitory (-) purine nucleotide products and PRPP activation. Purine nucleotide products also inhibit PRPP synthetase activity. Purine nucleotides and nucleosides are readily interconverted by means of an extensive and complex series of enzyme-catalyzed reactions that provide the cellular requirements for balanced availability of adenine and guanine nucleotides and nucleosides. Phosphorolysis of the nucleosides inosine and guanosine result in production of Hyp and Gua, which are either salvaged (in the HPRT reaction) or are ultimately and irreversibly oxidized through the base xanthine (Xan) to the end product, uric acid, in reactions catalyzed by xanthine oxidase.
