The history of the pulmonary artery catheter spans almost 90 years from the first reported cardiac catheterization by Werner Forssmann (on himself!) in 1929. Some 25 years later, a balloon-tipped catheter was developed by Lategola and Rahn [1] and used in dogs, but the name of the catheter (and much of the credit for its invention) went to Swan and Ganz, whose now famous paper on the use of a balloon-tipped catheter to catheterize the pulmonary artery was published in 1970 [2]. The innovative use of the balloon to guide the catheter made this a huge advance for cardiology and haemodynamic monitoring and management. Since that date, the pulmonary artery (Swan-Ganz) catheter (PAC) has changed little in size or structure, and has become one of the most widely used pieces of equipment in the intensive care unit (ICU).
However, in recent years, with the push to make medical care as noninvasive as possible and with the development of possible alternative, less invasive means of monitoring, the role of the PAC has come under close scrutiny. Intensivists are divided in their opinions, split into those who maintain that the haemodynamic data provided by the PAC aid in diagnosis and patient management, and those who believe that the complications and limitations outweigh the benefits. Increasingly, evidence does seem to suggest that patients managed with a PAC have similar outcomes to those without [3-7], although some studies have shown worse outcomes [8,9] and others improved outcomes [10]. The studies that have been conducted have used either complex statistical methodology to compare cohorts of patients [7,9,11] or have randomized patients to be managed with or without a PAC [3-6,12].
In a recent observational study conducted across Europe and including 3147 patients, a cohort of 481 patients who had a PAC inserted was compared with a cohort of patients with no PAC [7]. PAC use was not an independent risk factor for 60-day mortality in multivariate analysis, and in 453 propensity-matched pairs ICU and hospital mortality rates were similar between groups (PAC use versus no PAC use: ICU mortality 26.7% versus 26.3%; hospital mortality 31.4% versus 32.8%; not significant). In the most recent randomized study, 1041 ICU patients were randomly assigned to treatment with or without a PAC [6]. Physicians managing patients without a PAC were allowed to use alternative monitoring equipment (selected for 79% of patients) if they wished. There were no differences in hospital mortality (68% versus 66%; P = 0.039), hospital length of stay, or days of organ support between patients managed with and those managed without a PAC, and in a cost-effectiveness analysis the authors concluded that there would be considerable savings if the PAC were to be withdrawn from clinical use.
So, amidst all of these gloomy reports, does the PAC have a future or is it doomed to gather dust at the back of ICU equipment cupboards before reaching its final resting place as a curiosity in museums of medical history? I believe that the PAC still has a place in today's ICU, and that the information it provides can be integrated with that derived from newer equipment to optimize patient care. The PAC is a monitoring tool; if it is used to direct therapy and there is no improvement in outcome, then the therapy does not help. We know that PAC-derived data can prompt therapy to improve patient outcomes [13] but such improvements are not always achieved (e.g. sometimes physicians do not make the necessary changes to their therapy as suggested by the measurements) or indeed there may be overzealous application of therapies (e.g. fluid challenge for low cardiac filling pressure when there is no need for it). Thus, there is a need for better strategies based on the measurements obtained.
Many items of monitoring equipment widely used in the ICU have never undergone randomized controlled testing, including the electrocardiogram, yet we perform many electrocardiograms every day! Any tool is only as good as those who operate it. We need better, and continuing, training in haemodynamic monitoring, which has evolved over time (and continues to do so); initially filling pressures were monitored, then cardiac output measurements were added, and then mixed venous oxygen saturation (SvO2) measurements (and there are still people who do not measure SvO2). Today, we propose the reverse; treatment strategies should be directed first by SvO2, then by cardiac output and finally by pulmonary artery pressures [14] (Figure 1). We must also emphasize that PAC monitoring has evolved toward complete, 'continuous' measurements not only of cardiac output and ejection fraction but also of SvO2. This might dramatically influence our understanding of clinical situations as compared with past times when measurements obtained with a PAC were taken and considered only two or three times per day at best! It is difficult to imagine monitoring electrocardiography or pulse oximetry only two to three times per day. In my view, the ability to take continuous measurements of two or more crucial physiological parameters will make the PAC a unique monitoring tool for years to come – one that is very different from other techniques such as diagnostic echocardiography.
Figure 1.
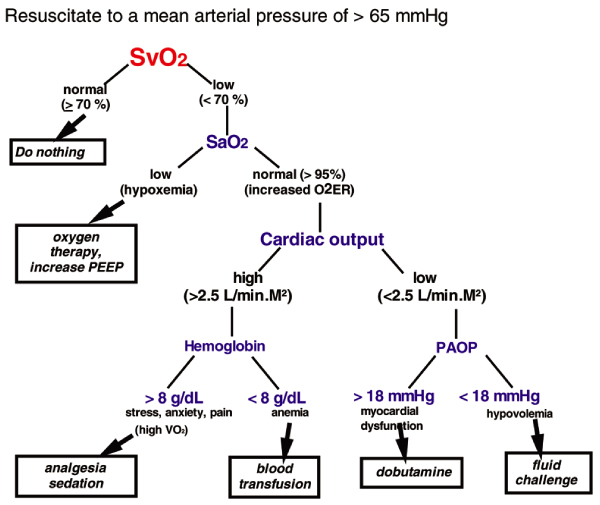
Suggested protocol for resuscitation using PAC-derived data. O2ER, oxygen extraction ratio; PAC, pulmonary artery catheter; PAOP, pulmonary artery occlusion pressure; PEEP, positive end-expiratory pressure; SaO2, arterial oxygen saturation; SvO2, mixed venous oxygen saturation; VO2, oxygen consumption. From Pinsky and Vincent [14], with permission.
In this supplement we explore some of the key issues surrounding the current and future role of the PAC and application of PAC-derived data in ICU patients; the reviews contained herein will, I believe, help as we struggle to integrate the PAC with newer technologies.
Abbreviations
ICU = intensive care unit; PAC = pulmonary artery catheter; SvO2 = mixed venous oxygen saturation.
Competing interests
JLV received honoraria and grants from Edwards Lifesciences, Pulsion and LiDCO.
References
- Lategola M, Rahn H. A self-guiding catheter for cardiac and pulmonary arterial catheterization and occlusion. Proc Soc Exp Biol Med. 1953;84:667–668. doi: 10.3181/00379727-84-20745. [DOI] [PubMed] [Google Scholar]
- Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man using a flow directed balloon tipped catheter. N Engl J Med. 1970;283:447–451. doi: 10.1056/NEJM197008272830902. [DOI] [PubMed] [Google Scholar]
- Rhodes A, Cusack RJ, Newman PJ, Grounds RM, Bennett ED. A randomised, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Med. 2002;28:256–264. doi: 10.1007/s00134-002-1206-9. [DOI] [PubMed] [Google Scholar]
- Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H. et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F. et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–2720. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K. PAC-Man study collaboration. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366:472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- Sakr Y, Vincent JL, Reinhart K, Payen D, Wiedermann CJ, Zandstra DF, Sprung CL. Use of the pulmonary artery catheter is not associated with worse outcome in the intensive care unit. Chest. 2006. in press . [DOI] [PubMed]
- Zion MM, Balkin J, Rosenmann D, Goldbourt U, Reicher-Reiss H, Kaplinsky E, Behar S. Use of pulmonary artery catheters in patients with acute myocardial infarction. Analysis of experience in 5,841 patients in the SPRINT Registry. SPRINT Study Group. Chest. 1990;98:1331–1335. doi: 10.1378/chest.98.6.1331. [DOI] [PubMed] [Google Scholar]
- Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM. et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA. 1996;276:889–897. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, McManus E. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ. 1999;318:1099–1103. doi: 10.1136/bmj.318.7191.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittock DR, Dhingra VK, Ronco JJ, Russell JA, Forrest DM, Tweeddale M, Fenwick JC. Severity of illness and risk of death associated with pulmonary artery catheter use. Crit Care Med. 2004;32:911–915. doi: 10.1097/01.CCM.0000119423.38610.65. [DOI] [PubMed] [Google Scholar]
- Yu DT, Platt R, Lanken PN, Black E, Sands KE, Schwartz JS, Hibberd PL, Graman PS, Kahn KL, Snydman DR. et al. Relationship of pulmonary artery catheter use to mortality and resource utilization in patients with severe sepsis. Crit Care Med. 2003;31:2734–2741. doi: 10.1097/01.CCM.0000098028.68323.64. [DOI] [PubMed] [Google Scholar]
- Mimoz O, Rauss A, Rekik N, Brun-Buisson C, Lemaire F, Brochard L. Pulmonary artery catheterization in critically ill patients: A prospective analysis of outcome changes associated with catheter-prompted changes in therapy. Crit Care Med. 1994;22:573–579. doi: 10.1097/00003246-199404000-00011. [DOI] [PubMed] [Google Scholar]
- Pinsky MR, Vincent JL. Let us use the PAC correctly and only when we need it. Crit Care Med. 2005;33:1119–1122. doi: 10.1097/01.CCM.0000163238.64905.56. [DOI] [PubMed] [Google Scholar]


