Abstract
Silymarin, derived from the milk thistle plant Silybum marianum and widely used for self-treatment of liver diseases, is composed of six major flavonolignans including silybin A and silybin B, which are the predominant flavonolignans quantified in human plasma. The single- and multiple-dose pharmacokinetics of silymarin flavonolignans were examined in patients with nonalcoholic fatty liver disease (NAFLD) or hepatitis C virus (HCV) to determine whether the disposition of silymarin and therefore its potential efficacy vary among liver disease populations. Cohorts of eight subjects with noncirrhotic liver disease were randomized 3:1 to oral silymarin or placebo (280 or 560 mg) every 8 h for 7 days. Forty-eight-hour blood sampling was conducted after the first and final doses. In general, plasma concentrations of silybin A and silybin B were higher, whereas concentrations of conjugates were lower in NAFLD compared with HCV. After adjustment of the area under plasma concentration-time curve from 0 to 8 h (AUC0–8 h) for weight and dose, only silybin B and silybin B conjugates differed significantly between disease types. For NAFLD, the adjusted mean AUC0–8 h was higher for silybin B (p < 0.05) but lower for silybin B conjugates (p < 0.05) compared with that for HCV. At the 280-mg dose, steady-state plasma concentrations of silybin B conjugates for NAFLD subjects were characterized by 46% lower AUC0–8 h (p < 0.05) and 42% lower Cmax (p < 0.05) compared with HCV subjects. Evidence of enterohepatic cycling of flavonolignans was only observed in NAFLD subjects. In summary, the efficacy of silymarin may be more readily observed in NAFLD patients because of their higher flavonolignan plasma concentrations and more extensive enterohepatic cycling compared with those in HCV patients.
Introduction
Silymarin is an herbal product that has been used for centuries for diseases of the liver (Flora et al., 1998), and approximately one-third of patients seen in U.S. liver clinics report the use of some complementary and alternative medicine to self-treat their liver disease (Strader et al., 2002). Derived from the milk thistle plant Silybum marianum, silymarin is a complex mixture of six major flavonolignans (silybins A and B, isosilybins A and B, silychristin, and silydianin), as well as other minor polyphenolic compounds (Kim et al., 2003). Silymarin has been shown to have antioxidant, anti-inflammatory/immunomodulatory, and antifibrotic properties in various in vitro and animal models (Abenavoli et al., 2010). However, the antioxidant activity of silymarin is most likely to attenuate the pathologic effects initiated by oxidative stress in the liver, which influence pathways of inflammation, necrosis, and fibrosis in chronic liver disease (Galli et al., 2005; Medina and Moreno-Otero, 2005).
Silymarin may be the most potent antioxidant in nature by virtue of its free radical scavenger reactivity and favorable membrane-lipid/water partitioning (György et al., 1992). Oxidative stress is thought to play a central role in the etiology of nonalcoholic steatohepatitis (NASH), a specific subset of nonalcoholic fatty liver disease (NAFLD), and is hypothesized to represent a “second hit” triggering the necroinflammatory response characteristic of NASH (Day and James, 1998). Therefore, the antioxidant properties of silymarin may be particularly beneficial as a treatment for NASH because patients have significantly increased levels of serum lipid peroxidation products (Chalasani et al., 2004) as well as other oxidative stress markers and decreased levels of antioxidant enzymes (Koruk et al., 2004). In addition, oxidative stress is a key feature of disease activity in HCV infection. Elevated levels of oxidative stress markers have been associated with the grade and stage of liver disease in HCV patients (Jain et al., 2002), which suggests that antioxidant therapy may be effective in slowing disease progression in the absence of antiviral effects. These observations provide the rationale for current phase 2 trials on the effects of silymarin in HCV and NASH populations.
The type and stage of liver disease have been recently shown to influence the single-dose pharmacokinetics of the major silymarin flavonolignans (Schrieber et al., 2008). An unexpected finding was that total silymarin flavonolignan exposures were 3- to 5-fold higher for patient cohorts compared with healthy controls (Schrieber et al., 2008). Whereas this study demonstrated that the pharmacokinetics of silymarin depend upon the type and grade/stage of liver disease, pharmacokinetic differences between patients with chronic HCV infection and NAFLD were not fully elucidated because of the low plasma concentrations.
Silymarin flavonolignans are metabolized via phase 2 conjugation pathways (Sridar et al., 2004; Jančová et al., 2011), and the majority of glucuronide and sulfate conjugates undergo hepatobiliary excretion via multidrug resistance protein (Mrp) 2 (Miranda et al., 2008). In obesity and NAFLD animal models, Mrp2 has been shown to have altered hepatic expression and function (Geier et al., 2005; Cheng et al., 2008). In addition, functional genetic polymorphisms in MRP2 have been associated with susceptibility to NAFLD and disease severity (Sookoian et al., 2009). Therefore, disease-specific modulation of silymarin-metabolizing enzymes or hepatic transporters may account for alterations in silymarin pharmacokinetics that have been previously observed in different types of liver diseases and therefore may have a profound effect on the efficacy in different patient populations.
We have previously reported on the ascending multiple dose pharmacokinetics of silymarin in noncirrhotic patients with chronic HCV infection (Hawke et al., 2010) obtained from a double-blind, placebo-controlled phase 1 trial that was conducted in patients with either HCV or NAFLD. An unexpected finding was that dose proportionality in the pharmacokinetics of parent silymarin flavonolignans was not observed in HCV patients with well compensated liver disease at silymarin doses greater than 560 mg when administered orally every 8 h (Hawke et al., 2010). Because the steady-state pharmacokinetics of silymarin in patients with NAFLD have not been described previously and because the pharmacokinetics of silymarin may be different in different types of liver diseases (Schrieber et al., 2008), we now report on the pharmacokinetics of silymarin in NAFLD subjects enrolled in the phase 1 trial. To determine whether the disposition of silymarin differs between patients with NAFLD or HCV liver disease, we also compare the single- and multiple-dose pharmacokinetics of silybin A and silybin B and their conjugates between patients with NAFLD or HCV. Finally, because the pharmacokinetics of silymarin appear to be nonlinear in patients with HCV, the pharmacokinetics of silymarin were evaluated at silymarin doses of 280 and 560 mg to assess the interaction between dose and disease type. These trials were conducted to optimize oral silymarin dosing for phase 2 efficacy trials in patients with either HCV or NASH (Lang, 2006). In these phase 2 trials, which are now ongoing, oral doses higher than the customary dose of 140 mg every 8 h are used in an attempt to overcome the high first-pass metabolism of silymarin and achieve therapeutic, steady-state plasma concentrations.
Materials and Methods
Subjects.
Forty male and female subjects ≥18 years of age with chronic noncirrhotic NAFLD and HCV were enrolled in the study within 28 days of screening (n = 8/cohort). Subjects were required to have elevated alanine aminotransferase levels ≥65 IU/l within 1 year before screening and a creatinine clearance (calculated according to the Cockcroft-Gault equation) >60 ml/min at screening as well as a negative urine pregnancy screen for women of child-bearing potential who were also required to use barrier methods of contraception during the study.
Subjects were excluded if they had either a history of or, in the clinical opinion of the investigators, evidence of decompensated liver disease defined by serum albumin <3.2 g/dl, total bilirubin >1.5 mg/dl, or prothrombin time/international normalized ratio >1.3 times normal or a history of or the presence of ascites, encephalopathy, portal hypertension, or bleeding from esophageal varices. Subjects were also excluded if they had evidence of other chronic liver disease or serologic evidence of infection with human immunodeficiency virus. Other exclusion criteria included an allergy to milk thistle or its preparations, use of silymarin or other milk thistle preparations, or use of high doses of other antioxidants such as vitamin E, vitamin C, glutathione, or α-tocopherol within 30 days of randomization through study completion. However, use of standard doses of over-the-counter multivitamins or cough/cold preparations was allowed. Also excluded was the chronic use of >2 g/day acetaminophen; use of oral contraceptives, warfarin, or metronidazole; or concurrent use of the following cytochrome CYP3A4 inducers: aminoglutethimide, aprepitant, carbamazepine, dexamethasone, efavirenz, ethosuximide, garlic supplements, glucocorticoids, glutethimide, griseofulvin, modafinil, nafcillin, nevirapine, oxcarbazepine, phenobarbital, phenytoin, primidone, rifabutin, rifampin, rifapentine, and St. John's wort; historical liver biopsy demonstrating the presence of cirrhosis (Ishak stage 5 or 6) or ≥15% steatosis or evidence of steatohepatitis; positive urine screen for drugs of abuse; alcohol consumption of >12 g/day for ≥6 months before screening; or other evidence of alcohol or drug abuse within 6 months of screening. Women who were pregnant or breast-feeding were also excluded. All subjects agreed not to consume alcohol for 48 h before study randomization through study completion.
Trial Design.
Specific details on the design of this phase 1 study have been described previously (Hawke et al., 2010). In brief, dose cohorts of eight subjects each were randomized 3:1, via a web-based randomization system used by each site's pharmacist, to receive oral silymarin or placebo every 8 h for 7 days. Forty-eight-hour pharmacokinetic samples were collected after an initial single-dose administration before the 7-day treatment and a final dose after the 7-day treatment for a total of 23 doses. Only pharmacists were unblinded to treatment assignments until trial completion. The sample size was selected to provide information on safety, tolerability, and pharmacokinetics of silymarin and was based on historical experience for phase 1 trials and not on statistical considerations. Cohorts were enrolled sequentially at doses of 280 or 560 mg of silymarin. The Legalon (Rottapharm|Madaus, Monza, Italy) brand of silymarin was selected as the clinical trial material for the Silymarin Product Development Program for use in National Institutes of Health-sponsored clinical trials for liver diseases from competing bids in response to a Notice of Opportunity by the National Center for Complementary and Alternative Medicine and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
The first and last doses for the pharmacokinetic studies were administered on days 1 and 10, respectively. To control for potential variability induced by fed versus fasted states, doses were administered with 240 ml of water 30 min after breakfast to subjects who were fasted overnight. Subjects were allowed to choose from a fixed list of items on the clinical research breakfast menu. Grapefruit juice was not allowed. Subjects remained in the research unit for 48 h for collection of blood. Fourteen serial blood samples were collected at 0 h (predose) and 0.5, 1, 1.5, 2, 4, 6, 8, 12, 15, 18, 24, 32, and 48 h postdose. Twenty-one doses were dispensed to subjects upon discharge after collection of the 48-h postdose sample on day 3. The first of these 21 doses was self-administered under direct supervision in the clinical research center. Eight-hour postdose trough plasma samples were collected during safety visits on days 6 and 8. Patient adherence was assessed by patient drug diary, by pill counts, and by maintaining records of drugs dispensed and returned.
Subjects were enrolled from December 2006 to July 2008 at four clinical centers, which included University of North Carolina at Chapel Hill, Beth Israel Deaconess Medical Center, University of Pennsylvania, and Thomas Jefferson University. Institutional review boards of participating centers approved the protocol; all subjects provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and guidelines on Good Clinical Practice.
Safety Assessment.
Safety, which consisted of clinical laboratory tests and reports of clinical adverse events using a symptom assessment questionnaire, was assessed before dosing on study days 1 (baseline), 6, 8, and 10. In addition, on days 1 and 10, the questionnaire was also completed at approximately 24 and 48 h postdose. Common Terminology Criteria for Adverse Events (version 3.0) was used to grade the severity of adverse events. Physical examinations and electrocardiograms were completed at baseline and at the end of the study. Decisions to dose escalate were made after a safety evaluation by a designated safety committee masked to treatment. The safety committee consisted of the principal investigators from the four clinical centers and an external safety monitor.
Study Drug.
Silymarin (Legalon) and matching placebo were manufactured in hard capsules by Madaus Rottapharm Group (Cologne, Germany); all study doses were administered from lot 0418901. Each dose consisted of five silymarin and/or placebo capsules packaged in single-use medicine dose cups. The flavonolignan content of each capsule was determined according to previously published liquid chromatography-mass spectrometry methods as follows: 23.2 mg, silybin A; 32.0 mg, silybin B; 11.8 mg, isosilybin A; 6.6 mg, isosilybin B; 24.9 mg, silychristin; and 29.0 mg, silydianin (Wen et al., 2008). These six flavonolignans account for 70.8% (127.5 mg of silymarin equivalent to 140 mg of silymarin as determined by the manufacturer's 2,4-dinitrophenylhydrazine method) of the 180-mg milk thistle extract contained in each capsule. Based on interim stability testing results performed by the manufacturer, Legalon capsules are stable under normal conditions (25°C, 60% relative humidity) for at least 9 months. For the purpose of the pharmacokinetic analyses described in this report, one Legalon capsule was considered equal to 140 mg of silymarin in accordance with the manufacturer's specifications.
Analysis of Silymarin Flavonolignans.
Whole-blood samples were collected in two 3-ml EDTA-lined tubes (K2-EDTA tubes; BD, Franklin Lakes, NJ) and centrifuged at 1200g for 10 min at 4°C. Plasma was aspirated and transferred to polypropylene tubes. Plasma samples were temporarily stored at −70°C by each clinical site for <30 days before shipment to the University of North Carolina where they were acidified by addition of glacial acetic acid (final concentration 1% acetic acid) and stored at −70°C until analysis.
For the determination of parent (i.e., nonconjugated) flavonolignan concentrations in plasma, a 125-μl aliquot of each patient sample was buffered using sodium acetate (pH 5.0, 0.125 M) and incubated for 6 h at 37°C without hydrolytic enzymes. A second 125-μl aliquot was also buffered using sodium acetate (pH 5.0, 0.125 M) and incubated with a mixture of sulfatase (80 U/ml, type H-1) and β-glucuronidase (8000 U/ml, type B-10) (Sigma-Aldrich, St. Louis, MO) for the determination of total (i.e., parent + conjugates) flavonolignan concentrations, which were expressed as “parent flavonolignan equivalents.” After incubation, 50 ng of naringenin (internal standard) in 25 μl of 50% methanol was added to the samples, which were then deproteinized and processed using a high-throughput protein filtration procedure as described previously (Hawke et al., 2010). After filtration, 75 μl of the plasma sample supernatants were transferred to glass high-performance liquid chromatography vials and concentrations of silymarin flavonolignans were quantified by liquid chromatography-electrospray ionization-mass spectrometry as described previously using a Luna C18 analytical column (50 × 2.0 mm i.d., 3 μm; Phenomenex, Torrance, CA); an isocratic mobile phase consisting of 43% methanol, 56% water, and 1% glacial acetic acid (pH 2.8); a flow rate of 0.3 ml/min; a 25-μl injection volume; and a 13-min run time (Wen et al., 2008). For each silymarin flavonolignan, the limit of detection was 20 ng/ml, and the quantitative ranges for parent and for total flavonolignan were 50 to 2500 and 100 to 20,000 ng/ml, respectively. The accuracy for each flavonolignan was within 95.4 to 107.4% and intra- and interday precisions were 1.7 to 11 and 4.5 to 14%, respectively.
Data Analysis.
Pharmacokinetic parameters including area under the plasma concentration-time curve (AUC), maximum plasma concentration (Cmax), time to Cmax (Tmax), and terminal half-life (t1/2) were calculated using noncompartmental methods (WinNonlin Professional version 5.2; Pharsight, Mountain View, CA). A constant dosing interval (τ) of 8 h was assumed for the calculation of steady-state AUC0–8 h using the linear up/log down trapezoidal method. To obtain pharmacokinetic parameters for the conjugate flavonolignan concentrations, the parent flavonolignan concentrations were subtracted from the total flavonolignan concentrations at each time point over the entire sampling period before pharmacokinetic analysis was performed. Pharmacokinetic parameters are reported as geometric means with 95% confidence intervals, except for Tmax, which is reported as median with minimum and maximum values. For our primary analysis, differences in steady-state exposures (i.e., AUC0–8 h) between disease cohorts were compared, after log transformation, using a parametric two-sample t test. p < 0.05 was used for statistical significance. In addition, to eliminate weight as a potential confounder in the assessment of differences in flavonolignan exposures between cohorts, a linear regression model with log AUC0–8 h as outcome was used. The model included dose, disease, and weight as independent variables to adjust for variable weights across dose groups (280 mg versus 560 mg) or disease type (HCV versus NAFLD) while comparing AUC0–8 h. Least-squares means (adjusted means) were reported with 95% confidence intervals and tested using t tests. All statistical analyses were performed by using SAS 9.2 or SAS JMP 9 (SAS Institute, Cary, NC).
Results
Subjects.
Baseline demographics are presented in Table 1. Study subjects in the HCV cohorts were predominantly men with ages ranging from 43 to 59 years, whereas men and women were more equally represented in the NAFLD cohorts with ages ranging from 28 to 58 years. Subjects were characterized by well compensated, noncirrhotic liver disease as evidenced by total bilirubin (range 0. 3–2.6 mg/dl) and platelet counts (range 150–327 cells/mm3).
TABLE 1.
Subject baseline demographics
Data are presented as medians (minimum, maximum).
| Group and Cohort |
||||
|---|---|---|---|---|
| HCV |
NAFLD |
|||
| 280 mga | 560 mg | 280 mg | 560 mg | |
| Male/female (n) | 8/4 | 5/1 | 3/3 | 2/4 |
| White/black (n) | 10/2 | 4/2 | 6/0 | 6/0 |
| Age (years) | 50 (44, 59) | 51 (43, 54) | 51 (28, 58) | 48 (28, 52) |
| Weight (kg) | 92 (67, 99) | 104 (86, 123) | 104 (79, 150) | 101 (78, 143) |
| BMI (kg/m2) | 28 (25, 42) | 33 (26, 41) | 38 (27, 42) | 38 (27, 43) |
| Total bilirubin (mg/dl) | 0.8 (0.3, 1.0) | 0.6 (0.3, 1.4) | 0.6 (0.3, 1.1) | 0.5 (0.3, 2.6) |
| ALT (U/l) | 95 (58, 288) | 113 (81, 214) | 99 (52, 322) | 104 (77, 115) |
| AST (U/l) | 64 (43, 271) | 89 (46, 110) | 60 (38, 325) | 66 (54, 104) |
| Platelets (cells/mm3) | 208 (177, 327) | 192 (150, 225) | 275 (157, 341) | 282 (162, 319) |
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Two cohorts of six subjects were used to study single and multiple dose pharmacokinetics.
Efficacy and Safety Endpoints.
Compared with their screening baseline values, no reductions in serum transaminases for either HCV or NAFLD subjects or reductions in HCV RNA titer for HCV subjects were observed at the end of the 7-day treatment period (data not shown).
There were no abnormal deviations from baseline laboratory values reported with silymarin administration for any cohort. For the HCV cohorts, three subjects who received a single 280-mg dose of silymarin reported a total of four adverse events. Three of the adverse events were classified as neurologic (e.g., headache), whereas the other was classified as gastrointestinal. Only one adverse event (dizziness) was considered possibly related to silymarin administration and resolved in less than 1 day.
For the NAFLD cohorts, 2 of 12 subjects (16.7%) receiving silymarin reported at least one adverse event compared with one of four subjects (25%) receiving placebo. Adverse events reported with silymarin included upper respiratory infection and abdominal pain, both of which occurred in the 560-mg dose cohort. All adverse events reported with silymarin were determined to be mild to moderate and self-limiting and were considered unrelated to treatment.
Single-Dose Pharmacokinetics of Silybin A and Silybin B.
A comparison of the pharmacokinetics of silybin A and silybin B between HCV and NAFLD cohorts after single oral doses of either 280 or 560 mg of silymarin are presented in Table 2. Silybin A was the predominant flavonolignan in plasma for both HCV and NAFLD cohorts and was characterized by a 2.7- to 3.3-fold greater Cmax and a 2- to 4.5-fold greater AUC0–48 h compared with those for silybin B.
TABLE 2.
Single-dose pharmacokinetics of parent silybin A and silybin B
Results are shown as geometric mean (95% confidence interval), except for Tmax, which is shown as median (minimum, maximum). Data are for n = 6 subjects.
| Cohort | PK Parameter | SA |
SB |
||
|---|---|---|---|---|---|
| HCV | NAFLD | HCV | NAFLD | ||
| 280 mg | AUC0–8 h (ng · h/ml) | 201 (115–338) | 228 (75–469)a | 93 (15–188)b | 80 (70–91)a |
| Cmax (ng/ml) | 78 (32–147) | 82 (35–153)a | 27 (8–50)b | 30 (16–53)a | |
| Tmax (h) | 2.0 (1.0, 4.0)b | 2.0 (1.0, 6.0)a | 3.0 (1.0, 4.0)b | 2.0 (2.0, 6.0)a | |
| t1/2 (h) | 1.3 (0.7–2.0) | 1.8 (−2.2–7.6) | 0.9 (−0.9–6.3)b | 1.8 (−1.6–6.6) | |
| 560 mg | AUC0–8 h (ng · h/ml) | 557 (470–657) | 859 (508–1397) | 125 (94–160)*,a | 261 (164–395) |
| Cmax (ng/ml) | 192 (147–250) | 275 (127–491) | 58 (31–92)a | 93 (55–145) | |
| Tmax (h) | 1.5 (1.0, 4.0) | 2.7 (1.5, 4.0) | 1.5 (0.5, 4.0)a | 2.7 (1.0, 4.0) | |
| t1/2 (h) | 1.4 (0.9–2.1) | 1.4 (0.8–2.3) | 1.1 (0.7–1.7)a | 1.5 (0.6–2.9) | |
SA, silymarin A; SB, silymarin B.
p < 0.05.
n = 5.
n = 4.
At the 280-mg dose, no differences were observed in the pharmacokinetics of silybin A or silybin B between HCV and NAFLD subjects. Short elimination half-lives were observed for both silybin A and silybin B (range 0.9–1.8 h).
However, at the 560-mg dose, pharmacokinetic differences were observed between subjects with HCV and NAFLD. Compared with HCV subjects, for NAFLD subjects, AUC0–48 h for silybin A and silybin B were 1.5-fold (p > 0.05) and 2.1-fold (p < 0.05) greater, respectively. A similar trend was observed in the Cmax for silybin A and silybin B, although the 1.4- to 1.6-fold differences between HCV and NAFLD subjects did not achieve statistical significance. Elimination half-lives were similar between the disease groups (range 1.1–1.5 h), whereas Tmax was delayed by 1 h in NAFLD subjects.
Steady-State Pharmacokinetics of Silybin A and Silybin B.
The steady-state pharmacokinetics of silybin A and silybin B for the HCV and NAFLD cohorts after chronic oral administration of either 280 or 560 mg of silymarin every 8 h for 7 days are presented in Table 3. Similar to the data obtained after single doses, silybin A was the predominant flavonolignan in plasma for both HCV and NAFLD cohorts and was characterized by a 2.1- to 3.6-fold greater Cmax and a 2.6- to 4.9-fold greater AUC0–8 h compared with those for silybin B. In addition, there was no evidence of accumulation for either flavonolignan after repeated dosing with elimination half-lives ranging between 0.7 and 1.3 h. Also similar to the single-dose data, pharmacokinetic differences between the HCV and NAFLD cohorts were only observed at the 560-mg dose. The AUC0–8 h for silybin A and silybin B were 1.6- and 2.5-fold greater, respectively, in NAFLD subjects than in HCV subjects at the 560-mg dose, whereas differences in the Cmax between cohorts ranged between 1.5- and 2.2-fold. After adjustment for weight and disease type, silybin A and silybin B AUC0–8 h differed significantly between the 280- and 560-mg dose groups (p ≤ 0.004), such that for either HCV or NAFLD or at any weight level, the 560-mg dose was associated with higher AUC0–8 h. With adjustment for weight and dose, only silybin B differed significantly across disease types such that the adjusted mean AUC0–8 h for silybin B was higher for NAFLD than for HCV (p = 0.004). The higher silybin B exposures in NAFLD subjects suggest the metabolism or hepatic uptake of silybin B may be reduced in NAFLD compared with HCV.
TABLE 3.
Steady-state pharmacokinetics of silybin A and silybin B
Results are shown as geometric mean (95% confidence interval), except for Tmax, which is shown as median (minimum, maximum). Data are for n = 6 subjects, except for the HCV 560-mg steady-state cohort where n = 5; one subject was dropped from the pharmacokinetic analysis because of incorrect dosing for pharmacokinetic sampling at steady-state on day 8.
| Cohort | PK Parameter | SA |
SB |
||
|---|---|---|---|---|---|
| HCV | NAFLD | HCV | NAFLD | ||
| 280 mg | AUC0–8 h (ng · h/ml) | 370 (279–480) | 317 (191–499) | 86 (66–109) | 123 (53–219)a |
| Cmax (ng/ml) | 143 (78–242) | 133 (60–262) | 45 (28–72) | 64 (30–111)a | |
| Tmax (h) | 1.8 (1.0, 4.0) | 1.3 (0.5, 4.4) | 1.5 (0.5, 4.0) | 1.2 (0.5, 2.0)a | |
| t1/2 (h) | 1.1 (0.7–1.6) | 1.1 (0.3–2.6) | 0.7 (0.4–1.2)a | 0.9 (0.2–2.1)a | |
| 560 mg | AUC0–8 h (ng · h/ml) | 729 (371–1195) | 1166 (589–2128) | 149 (40–310) | 376 (161–759) |
| Cmax (ng/ml) | 308 (104–620) | 448 (255–724) | 86 (26–186) | 187 (93–332) | |
| Tmax (h) | 1.5 (1.5, 2.0) | 3.0 (0.5, 4.0) | 1.5 (1.5, 2.0) | 3 (0.5, 4.0) | |
| t1/2 (h) | 1.3 (0.9–1.9) | 1.0 (0.6–1.6) | 1.1 (0.3–2.2) | 0.7 (0.4–1.0) | |
SA, silymarin A; SB, silymarin B.
n = 5.
Single-Dose and Steady-State Pharmacokinetics of Silybin A and Silybin B Conjugates.
To further explore the effect of NAFLD on metabolism of silymarin, differences in the plasma concentrations of silybin A and silybin B conjugates between HCV and NAFLD subjects were examined. As defined under Materials and Methods, plasma concentrations of conjugates were estimated from subtraction of parent flavonolignan concentrations from total (parent + conjugate) flavonolignan concentrations.
The single-dose and steady-state pharmacokinetic data for total conjugates of silybin A and silybin B for both disease cohorts are presented in Tables 4 and 5, respectively. Whereas plasma concentrations were observed to be greater for silybin A than for silybin B, the converse was true for their conjugates. The Cmax and AUC0–8 h for silybin B conjugates were 3- to 4-fold greater than those for silybin A conjugates across both dose levels and disease cohorts.
TABLE 4.
Single-dose pharmacokinetics of silybin A conjugates and silybin B conjugates
Results are shown as geometric means (95% confidence interval), except for Tmax, which is shown as median (minimum, maximum). Data are for n = 6 subjects.
| Cohort | PK Parameter | SAconjugates |
SBconjugates |
||
|---|---|---|---|---|---|
| HCV | NAFLD | HCV | NAFLD | ||
| 280 mg | AUC0–48 h (ng · h/ml) | 1327 (860–1925) | 1003 (672–1456) | 4094 (2465–6308) | 3120 (2247–4233) |
| Cmax (ng/ml) | 144 (96–205) | 80 (45–125) | 586 (441–762) | 388 (266–544) | |
| Tmax (h) | 2 (1.5, 4.0) | 4.0 (2.0, 12.0) | 2.0 (1.5, 4.0) | 3.0 (2.0, 8.0) | |
| t1/2 (h) | 5.7 (4.0–7.7) | 6.4 (4.5–9.0) | 4.3 (3.1–5.6) | 4.5 (2.5–7.2) | |
| 560 mg | AUC0–48 h (ng · h/ml) | 3468 (1747–6024) | 2844 (1493–4779) | 12,760 (6505–22,432) | 7850 (4501–12,664) |
| Cmax (ng/ml) | 339 (169–621) | 278 (126–488) | 1691 (1003–2774) | 1125 (805–1570) | |
| Tmax (h) | 3.0 (2.0, 4.0) | 4.0 (4.0, 6.2) | 4.0 (1.0, 4.0) | 3.0 (1.5, 6.0) | |
| t1/2 (h) | 6.6 (4.5–9.2) | 7.0 (5.0–9.7) | 4.7 (3.7–6.0) | 6.2 (3.3–10.3) | |
SA, silymarin A; SB, silymarin B.
TABLE 5.
Steady-state pharmacokinetics of silybin A conjugates and silybin B conjugates
Results are shown as geometric means (95% confidence interval), except for Tmax, which is shown as median (minimum, maximum). Data are for n = 6 subjects, except for the HCV 560-mg steady-state cohort where n = 5; one subject was dropped from the pharmacokinetic analysis because of incorrect dosing for pharmacokinetic sampling at steady state on day 8.
| Cohort | PK Parameter | SAconjugates |
SBconjugates |
||
|---|---|---|---|---|---|
| HCV | NAFLD | HCV | NAFLD | ||
| 280 mg | AUC0–8 h (ng · h/ml) | 2048 (1465–2815) | 1297 (652–2271) | 7278 (5633–9287)* | 3962 (2338–6292) |
| Cmax (ng/ml) | 369 (294–459)* | 233 (146–352) | 1294 (1040–1589)* | 750 (563–981) | |
| Tmax (h) | 2 (1, 4) | 2 (0, 4) | 2 (1, 4) | 1.8 (0, 2) | |
| t1/2 (h) | 5 (3–8.3) | 6.8 (2.5–15.5) | 4.3 (2.8–6.3) | 5.1 (1.2–12.7) | |
| 560 mg | AUC0–8 h (ng · h/ml) | 3229 (1618–5348) | 2902 (1403–5163) | 11003 (3244–21,930) | 7745 (4312–12,547) |
| Cmax (ng/ml) | 543 (182–1050) | 529 (287–894) | 2074 (700–4103) | 1592 (927–2600) | |
| Tmax (h) | 2 (0, 4) | 4 (0, 6) | 2 (2, 4) | 3 (0, 4) | |
| t1/2 (h) | 6.2 (3.6–9.3) | 3.6 (2.7–4.7) | 4.1 (2.9–5.6) | 2.8 (1.9–3.9) | |
SA, silymarin A; SB, silymarin B.
p < 0.05.
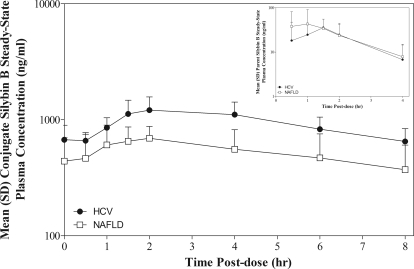
Differences between HCV and NAFLD subjects were observed in the pharmacokinetics for plasma conjugates of silybin A and silybin B at either dose level after single or chronic dosing. However, these differences only achieved significance between HCV and NAFLD cohorts dosed at 280 mg every 8 h, whereas conjugates of silybin B in plasma of NAFLD subjects were characterized by 46% lower AUC0–8 h (p < 0.05) and 42% lower Cmax (p < 0.05) compared with HCV subjects. Figure 1 depicts the mean steady-state plasma concentration versus time profiles for silybin B (inset) and silybin B conjugates for HCV and NAFLD subjects at the 280-mg dose. Plasma concentrations of silybin B conjugates were lower in NAFLD subjects compared with HCV subjects over the entire 8-h dosing interval (Fig. 1). In contrast, plasma concentrations of silybin B were higher in NAFLD subjects until peak concentrations were achieved and then declined similarly (Fig. 1, inset). These data suggest that reduced silymarin metabolism may result in differences in silymarin exposures between NAFLD and HCV subjects, rather than differences in absorption.
Fig. 1.
Steady-state plasma concentration versus time profiles for silybin B conjugates and parent silybin B (inset) at 280 mg of silymarin in HCV (●) and NAFLD (□) subjects. Forty-eight-hour plasma samples were obtained after a final single-dose administration after an every 8 h for 7-day dose regimen. AUC0–8 h and Cmax for silybin B conjugates were 46 and 42% lower, respectively, in NAFLD subjects compared with HCV; p < 0.05.
After adjustment for weight and disease type, the AUC0–8 h values for silybin A conjugates and for silybin B conjugates differed significantly between the 280- and 560-mg dose groups (p ≤ 0.004), such that for either HCV or NAFLD or at any weight level, the 560-mg dose was associated with higher AUC0–8 h. With adjustment for weight and dose, only silybin B conjugates differed significantly across disease types such that the adjusted mean AUC0–8 h for silybin B conjugates was significantly lower for NAFLD compared with HCV (p = 0.03).
To further quantify differences in the extent of flavonolignan conjugation between HCV and NAFLD subjects, steady-state metabolic ratios were calculated as the ratio of AUC0–8 h for silybin B divided by AUC0–8 h for silybin B conjugates at the 560-mg dose. Metabolic ratios differed 4-fold (p < 0.05) between HCV and NAFLD with means ± S.D. of 0.016 ± 0.011 and 0.060 ± 0.041, respectively. These data suggest that there is less conjugation of silybin B in NAFLD subjects then in HCV subjects at a silymarin dose of 560 mg. In summary, plasma concentrations of silybin A and silybin B were generally greater and the concentrations of their conjugates were lower in NAFLD subjects than in HCV subjects irrespective of the dose and frequency of oral silymarin administration.
Flavonolignan Accumulation.
The ratio of parent silybin A steady-state AUC0–8 h divided by single-dose AUC0–8 h was calculated as an indication of the extent of accumulation after chronic three times daily dosing. Silybin A ratios of 1.3 and 1.4 were calculated for HCV and NAFLD, respectively, at the 560-mg dose, which indicates no significant accumulation in either cohort with repeated dosing. Similar ratios were calculated for silybin B. This finding is consistent with the short half-life of the silymarin flavonolignans.
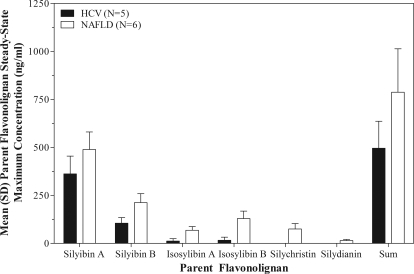
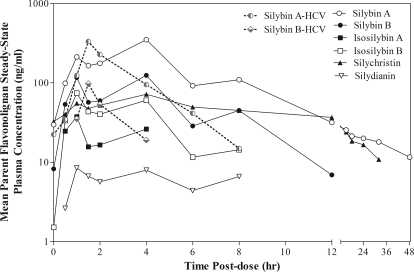
Although no evidence for parent silybin A and silybin B accumulation was observed, the overall amount of parent flavonolignans in plasma was significantly higher in NAFLD subjects than in HCV subjects at the 560-mg dose because of the appearance of additional parent flavonolignans. Figure 2 compares mean steady-state peak plasma concentrations of the six parent silymarin flavonolignans for HCV and NAFLD subjects at the 560-mg dose, as well as their sum concentration. As seen in Fig. 2, plasma concentrations of isosilybin A, isosilybin B, silychristin, and silydianin were significantly greater in NAFLD subjects than in HCV subjects. Of interest, silychristin and silydianin were not detected in the plasma of HCV subjects. To gain insight into the mechanism(s) behind these observed differences, we evaluated the plasma concentration versus time profile for each flavonolignan over the 48-h sampling period after administration of the last 560-mg dose (Fig. 3). Significant enterohepatic cycling of the six flavonolignans was observed in NAFLD subjects as indicated by a prominent second peak at 4 h after the absorption peak at 1 h. Most flavonolignans also showed evidence of a third peak at 8-h postdose. In contrast, there was less evidence of enterohepatic cycling in HCV subjects in whom no secondary peaks were observed for either silybin A or silybin B after the early absorption peak. Silychristin represented a major flavonolignan in the plasma of NAFLD subjects at the 560-mg dose. The steady-state pharmacokinetics of silychristin (geometric mean and 95% confident intervals) were characterized by a Cmax of 67 ng/ml (−2.5 to 174), an AUC0–8 h of 325 ng · h/ml (−145 to 1100), and a t1/2 of 3.1 h (1.2–6.3). The steady-state pharmacokinetics of the conjugates of silychristin in NAFLD subjects were characterized by a Cmax of 663 ng/ml (367–1394), an AUC0–8 h of 3800 ng · h/ml (1628–8462), and a t1/2 of 4.5 h (2.2–8.6).
Fig. 2.
Maximum steady-state plasma concentrations for silymarin flavonolignans at 560 mg of silymarin in HCV (■) and NAFLD (□) subjects. Plasma concentrations of isosilybin A, isosilybin B, silychristin, and silydianin were significantly greater in NAFLD subjects compared with HCV subjects. Silychristin and silydianin were not detected in the plasma of HCV subjects.
Fig. 3.
Steady-state plasma concentration versus time profiles for silymarin flavonolignans at 560 mg of silymarin in HCV and NAFLD subjects. Forty-eight-hour plasma samples were obtained after a final single dose administration after an every 8 h for 7-day dose regimen. Evidence of enterohepatic recycling of flavonolignans by the appearance of secondary peaks was observed in NAFLD subjects (——), whereas no evidence of enterohepatic recycling for silybin A or silybin B was observed in HCV subjects (– – –). In addition to silybin A and silybin B, silychristin (▴) represented a major flavonolignan in NAFLD subjects. For presentation clarity, error bars were not included.
Discussion
The expression of drug disposition genes and their protein products has been shown to be altered in liver disease (Congiu et al., 2002, 2009; Fisher et al., 2009), and effects of liver disease on the disposition of drugs have been demonstrated and tend to be more severe in patients with more advanced cirrhotic disease (Chalon et al., 2003). In contrast, significant differences in the disposition of drugs between different types of liver disease have not been demonstrated. We have shown that the disposition of silymarin, an herbal medicine widely used by patients with liver disease, is significantly altered in patients with liver disease (Schrieber et al., 2008). Concentrations of total silymarin species found in plasma, which consist primarily of flavonolignan conjugates, were found to be approximately 5-fold higher in patients with chronic HCV infection or NAFLD compared with those in healthy controls. Pharmacokinetic differences were also observed between healthy subjects and patients with NAFLD or patients with HCV cirrhosis. In contrast, differences were not observed between healthy subjects and patients with noncirrhotic HCV disease possibly due to wide disease heterogeneity in patient cohorts or reduced sensitivity as a result of low plasma concentrations of flavonolignans associated with the low oral dose of a generic brand of silymarin that was used in this study (Schrieber et al., 2008). These results raised the possibility that the disposition of silymarin and its potential beneficial effects may be different in various liver disease populations with early-stage disease. To determine whether the disposition of silymarin is different among patients with different types of the liver disease, this study examined the pharmacokinetics of higher than customary oral doses of silymarin in noncirrhotic patients with either chronic HCV infection or NAFLD. The results of our study show that NAFLD patients are characterized by higher plasma concentrations of certain silymarin flavonolignans and lower concentrations of flavonolignan conjugates compared with HCV patients administered the same dose. Although silymarin flavonolignans appear to share common pathways of metabolism and transport, differences in their affinity for these processes have been noted (Sridar et al., 2004; Miranda et al., 2008), which probably account for the different relationships between AUC exposure and dose for silybin A and silybin B observed in our study.
In vitro and in vivo studies suggest that silymarin flavonolignans are primarily metabolized through glucuronidation and sulfation pathways with various UDP-glucuronosyltransferases (UGTs) sharing overlapping specificity (Sridar et al., 2004; Jančová et al., 2011). In addition, the extent to which various flavonolignans undergo glucuronidation or sulfation appears to vary (Wen et al., 2008). There are several possibilities that could explain why the ratio of parent flavonolignan (e.g., silybin B) to flavonolignan conjugates was higher in patients with NAFLD than in those with HCV in our study. The simplest explanation is that the expression or activity of UGTs is decreased in NAFLD subjects. Nonalcoholic steatohepatitis, a specific subset of NAFLD, is characterized by hepatic steatosis and varying degrees of inflammation, which can lead to decreased UGT expression as has been observed in rodents (Richardson et al., 2006) and in human liver tissue (Congiu et al., 2002). Therefore, it is plausible that the major UGT isoforms involved in metabolism of silymarin may be lower in NAFLD subjects, resulting in higher plasma levels of parent flavonolignans and lower concentrations of conjugates. Because silybin B conjugates represent 99% of the total (parent + conjugates) silybin B species in plasma of patients with HCV, metabolism stoichiometry predicts that the 40% reduction in silymarin conjugates observed in our NAFLD cohort should result in an ∼30-fold increase in silybin B plasma concentrations. However, plasma concentrations of silybin B were comparable between HCV and NAFLD patients. Therefore, reduced UGT activity does not appear to be a viable explanation for the differences in silymarin pharmacokinetics between HCV and NAFLD in our study. In addition, the lower plasma concentration of flavonolignan conjugates in NAFLD compared with HCV does not appear to be related to reduced intestinal absorption because parent flavonolignans would also be expected to be lower in plasma.
Alterations in the expression and function of hepatobiliary transporters may be a more plausible explanation for the decrease in flavonolignan conjugates and the higher plasma concentrations of parent flavonolignans observed in the NAFLD cohorts. Evidence for extensive enterohepatic cycling of silymarin and their conjugates has been observed at high doses of silymarin (Schrieber et al., 2008; Hawke et al., 2010). Enterohepatic cycling is regulated by hepatobiliary transporters involved in the active uptake of anionic and cationic compounds from the blood such as the organic anion-transporting polypeptides, OATP1B1 and OATP2B1, located on the basolateral membrane of the hepatocyte (Chandra and Brouwer, 2004). In many instances, these compounds undergo metabolism to more polar conjugates followed by transport and biliary excretion by ATP-binding cassette transporters such as P-glycoprotein, MRP2, and breast cancer resistance protein, located at the canalicular membrane of the hepatocyte (Schinkel and Jonker, 2003; Leslie et al., 2005). Once delivered to the small intestine, parent compounds can be reformed by bacterial deconjugation and returned to portal blood for delivery to the liver for reuptake. In competition with biliary efflux is the efflux of substrates from the hepatocyte to blood by other members of the MRP family, such as MRP3 and MRP4 (MRPs 3/4), which are located on the basolateral (sinusoidal) membrane. It is generally thought that MRP2 and MRP3 work in concert in liver disease to promote hepatic efflux and protect the hepatocyte from the effects of cholestasis (Wagner et al., 2005; van de Steeg et al., 2010).
The most intriguing observation in the current study was the suggestion of significant enterohepatic recycling of silymarin flavonolignans in NAFLD subjects in contrast with HCV subjects in whom there was no evidence of enterohepatic cycling (Fig. 3). Silymarin flavonolignans demonstrate high affinity for MRP4 (Wu et al., 2005), whereas silymarin conjugates but not parent flavonolignans appear to be better substrates for MRP2 (Miranda et al., 2008). Glucuronides that are substrates for MRP2, such as conjugated bilirubin, can also be substrates for MRPs 3/4 (Borst et al., 2006; Zelcer et al., 2006). Therefore, differences in the disposition and enterohepatic cycling of silymarin flavonolignans may reflect alterations in the function of hepatobiliary transporters as a result of liver disease.
In obesity and NAFLD animal models, Mrp2 has been shown to have altered hepatic expression and function (Geier et al., 2005; Cheng et al., 2008). In addition, Mrp2, Mrp3, and Mrp4 protein expression were significantly increased in a rodent model of NAFLD (Lickteig et al., 2007). The biliary excretion of glucuronide and sulfate conjugates of silymarin flavonolignans was shown to be dependent on Mrp2 using isolated perfused livers, and some flavonolignans such as silychristin and silydianin were almost quantitatively secreted into bile (Miranda et al., 2008). Therefore, enterohepatic cycling of silymarin flavonolignans may be increased in NAFLD due to increased MRP2-dependent biliary efflux and diversion of silymarin conjugates away from sinusoidal efflux to blood. An increase in MRP4 would also contribute to greater sinusoidal efflux of parent flavonolignans. These changes would result in lower plasma concentrations of silymarin conjugates with higher concentrations of recycling silymarin flavonolignans in patients with NAFLD compared with those with HCV.
As an alternative, the differences observed in the disposition of silymarin between NAFLD and HCV patients may reflect HCV-specific alterations in hepatobiliary function. HCV infection was shown to be associated with increased hepatic expression of MRP4, decreased expression of MRP2, and decreased expression of OATP1B1 in cirrhotic and noncirrhotic liver, whereas the expression of MRP3 and OATP2B1 was similar to that in normal human liver (Ogasawara et al., 2010). Therefore, the differences in the disposition of silymarin between HCV and NAFLD subjects observed in our study may reflect a diversion of silymarin conjugates to sinusoidal efflux in HCV patients due to reduced biliary efflux by MRP2 or reduced uptake by OATP1B1, which would also result in higher plasma concentrations of silymarin conjugates and decreased enterohepatic cycling of silymarin flavonolignans compared with those in patients with NAFLD. Although the results of our study cannot delineate between these various potential mechanisms, it is possible that the disposition of silymarin is altered by different, disease-specific mechanisms in NAFLD and HCV populations. This conclusion is supported by our previous observation that plasma concentrations of silymarin conjugates are significantly higher in both NAFLD and HCV patients compared with concentrations found in healthy volunteers (Schrieber et al., 2008).
In summary, differences in the disposition of silymarin between NAFLD and HCV patients may reflect different disease-specific alterations in the function of hepatobiliary transport proteins. These observations are significant because differences in the disposition of drugs between different types of liver disease have not been demonstrated, perhaps because of their more restrictive use indications. Of importance, the antioxidant activity and potential anti-inflammatory and antifibrotic effects of silymarin on disease progression will be dependent on its hepatic disposition. Oxidative stress has been associated with all stages of chronic HCV liver disease (Jain et al., 2002), and recent data from the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial suggest that silymarin use among patients with advanced HCV liver disease may be associated with reduced progression to cirrhosis (Freedman et al., 2011). Silymarin may demonstrate greater benefits in patients with NAFLD compared with those with HCV infection because oxidative stress is thought to play a central role in the etiology of NASH (Day and James, 1998) and there are no approved therapies. In addition, the results of this study suggest that the effects of silymarin on liver disease progression may also be greater in NAFLD patients that in HCV patients because of higher flavonolignan plasma concentrations and more extensive enterohepatic cycling. These observations were critical in the design of a phase 2 silymarin trial in NASH, which is currently ongoing (Lang, 2006).
Acknowledgments
We are indebted to Drs. Josh Berman and Qi-Ying Liu for their important early efforts in study design and to Dr. Ulrich Mengs for championing this work. In addition, we thank the patients who volunteered for this trial, Dr. Tedi Soule, Joseph Colagreco, Mary Hammond, and Deborah Moretti, who served as the study coordinators, and Sharon Lawlor, who was the Data Coordinating Center coordinator, for their invaluable assistance in the conduct of this trial. We also thank Dr. Craig W. Hendrix, who graciously agreed to serve as the independent safety monitor.
This work was supported by Cooperative Agreements from the National Institutes of Health National Center for Complementary and Alternative Medicine [Grants U01-AT003571, U01-AT003560, U01-AT003573, U01-AT003566, U01-AT003574], with cofunding from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; and the National Institutes of Health National Center for Research Resources [Grant RR00046] (General Clinical Research Centers program). In addition, Rottapharm|Madaus, Italy, provided silymarin and placebo and partly funded the trial.
This was an investigator-initiated trial, and Rottapharm|Madaus had no direct or indirect involvement in the design of the trial, data collection, preparation, or submission of the manuscript for this registered (http://clinicaltrials.gov/ct2/show/NCT00389376) investigator-initiated trial. None of the authors have a personal conflict of interest with the manufacturer of any of the marketed silymarin formulations. No official endorsement by the U.S. Food and Drug Administration is intended or should be inferred.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/dmd.111.040212.
- NASH
- nonalcoholic steatohepatitis
- NAFLD
- nonalcoholic fatty liver disease
- HCV
- hepatitis C virus
- Mrp/MRP
- multidrug resistance protein
- AUC
- area under the plasma concentration-time curve
- UGT
- UDP-glucuronosyltransferase
- OATP
- organic anion-transporting polypeptide.
Authorship Contributions
Participated in research design: Hawke, Reddy, Belle, Afdhal, Navarro, Meyers, Doo, and Fried.
Conducted experiments: Schrieber and Wen.
Contributed new reagents or analytic tools: Hawke and Smith.
Performed data analysis: Schrieber and Wahed.
Wrote or contributed to the writing of the manuscript: Schrieber and Hawke.
References
- Abenavoli L, Capasso R, Milic N, Capasso F. (2010) Milk thistle in liver diseases: past, present, future. Phytother Res 24:1423–1432 [DOI] [PubMed] [Google Scholar]
- Borst P, Zelcer N, van de Wetering K. (2006) MRP2 and 3 in health and disease. Cancer Lett 234:51–61 [DOI] [PubMed] [Google Scholar]
- Chalasani N, Deeg MA, Crabb DW. (2004) Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 99:1497–1502 [DOI] [PubMed] [Google Scholar]
- Chalon SA, Desager JP, Desante KA, Frye RF, Witcher J, Long AJ, Sauer JM, Golnez JL, Smith BP, Thomasson HR, et al. (2003) Effect of hepatic impairment on the pharmacokinetics of atomoxetine and its metabolites. Clin Pharmacol Ther 73:178–191 [DOI] [PubMed] [Google Scholar]
- Chandra P, Brouwer KL. (2004) The complexities of hepatic drug transport: current knowledge and emerging concepts. Pharm Res 21:719–735 [DOI] [PubMed] [Google Scholar]
- Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, Slitt AL. (2008) Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm 5:77–91 [DOI] [PubMed] [Google Scholar]
- Congiu M, Mashford ML, Slavin JL, Desmond PV. (2002) UDP glucuronosyltransferase mRNA levels in human liver disease. Drug Metab Dispos 30:129–134 [DOI] [PubMed] [Google Scholar]
- Congiu M, Mashford ML, Slavin JL, Desmond PV. (2009) Coordinate regulation of metabolic enzymes and transporters by nuclear transcription factors in human liver disease. J Gastroenterol Hepatol 24:1038–1044 [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114:842–845 [DOI] [PubMed] [Google Scholar]
- Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. (2009) Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos 37:2087–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora K, Hahn M, Rosen H, Benner K. (1998) Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol 93:139–143 [DOI] [PubMed] [Google Scholar]
- Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE, and HALT-C Trial Group (2011) Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment Pharmacol Ther 33:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, et al. (2005) Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology 41:1074–1084 [DOI] [PubMed] [Google Scholar]
- Geier A, Dietrich CG, Grote T, Beuers U, Prüfer T, Fraunberger P, Matern S, Gartung C, Gerbes AL, Bilzer M. (2005) Characterization of organic anion transporter regulation, glutathione metabolism and bile formation in the obese Zucker rat. J Hepatol 43:1021–1030 [DOI] [PubMed] [Google Scholar]
- György I, Antus S, Blázovics A, Földiák G. (1992) Substituent effects in the free radical reactions of silybin: radiation-induced oxidation of the flavonoid at neutral pH. Int J Radiat Biol 61:603–609 [DOI] [PubMed] [Google Scholar]
- Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, et al. (2010) Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol 50:434–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A, Warnes TW. (2002) Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol 36:805–811 [DOI] [PubMed] [Google Scholar]
- Jančová P, Siller M, Anzenbacherová E, Křen V, Anzenbacher P, Simánek V. (2011) Evidence for differences in regioselective and stereoselective glucuronidation of silybin diastereomers from milk thistle (Silybum marianum) by human UDP-glucuronosyltransferases. Xenobiotica 41:743–751 [DOI] [PubMed] [Google Scholar]
- Kim NC, Graf TN, Sparacino CM, Wani MC, Wall ME. (2003) Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org Biomol Chem 1:1684–1689 [DOI] [PubMed] [Google Scholar]
- Koruk M, Taysi S, Savas MC, Yilmaz O, Akcay F, Karakok M. (2004) Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann Clin Lab Sci 34:57–62 [PubMed] [Google Scholar]
- Lang I. (2006) Phase I/II clinical trial to explore silymarin in chronic liver diseases. Gastroenterol Hepatol News 131:990 [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. (2005) Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204:216–237 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978 [DOI] [PubMed] [Google Scholar]
- Medina J, Moreno-Otero R. (2005) Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs 65:2445–2461 [DOI] [PubMed] [Google Scholar]
- Miranda SR, Lee JK, Brouwer KL, Wen Z, Smith PC, Hawke RL. (2008) Hepatic metabolism and biliary excretion of silymarin flavonolignans in isolated perfused rat livers: role of multidrug resistance-associated protein 2 (Abcc2). Drug Metab Dispos 36:2219–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K, Terada T, Katsura T, Hatano E, Ikai I, Yamaoka Y, Inui K. (2010) Hepatitis C virus-related cirrhosis is a major determinant of the expression levels of hepatic drug transporters. Drug Metab Pharmacokinet 25:190–199 [DOI] [PubMed] [Google Scholar]
- Richardson TA, Sherman M, Kalman D, Morgan ET. (2006) Expression of UDP-glucuronosyltransferase isoform mRNAs during inflammation and infection in mouse liver and kidney. Drug Metab Dispos 34:351–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55:3–29 [DOI] [PubMed] [Google Scholar]
- Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. (2008) The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos 36:1909–1916 [DOI] [PubMed] [Google Scholar]
- Sookoian S, Castaño G, Gianotti TF, Gemma C, Pirola CJ. (2009) Polymorphisms of MRP2 (ABCC2) are associated with susceptibility to nonalcoholic fatty liver disease. J Nutr Biochem 20:765–770 [DOI] [PubMed] [Google Scholar]
- Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. (2004) Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab Dispos 32:587–594 [DOI] [PubMed] [Google Scholar]
- Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, Allen J, Khokar MF, Hoofnagle JH, Seeff LB. (2002) Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol 97:2391–2397 [DOI] [PubMed] [Google Scholar]
- van de Steeg E, Wagenaar E, van der Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE, Schinkel AH. (2010) Organic anion transporting polypeptide 1a/1b-knockout mice provide insights into hepatic handling of bilirubin, bile acids, and drugs. J Clin Invest 120:2942–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42:420–430 [DOI] [PubMed] [Google Scholar]
- Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. (2008) Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos 36:65–72 [DOI] [PubMed] [Google Scholar]
- Wu CP, Calcagno AM, Hladky SB, Ambudkar SV, Barrand MA. (2005) Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5). FEBS J 272:4725–4740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, de Waart R, Scheffer GL, Marschall HU, Wielinga PR, Kuil A, Kunne C, Smith A, van der Valk M, et al. (2006) Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol 44:768–775 [DOI] [PubMed] [Google Scholar]