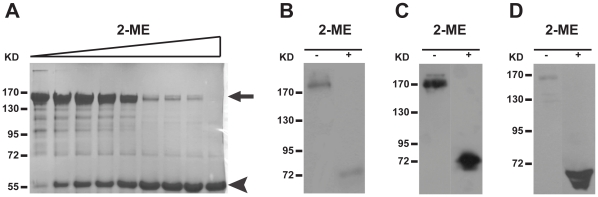
Figure 6. GOLPH2 forms disulfide bond-linked oligomers.
A, GOLPH2-Δ(1–55) was purified by Ni-NTA affinity chromatography, and then boiled to a final concentration of 0 mM to 100 mM 2-mercaptoethanol. Separation by 10% SDS-PAGE followed. GOLPH2 oligomers dissociated with increased 2-mercaptoethanol. B, The HeLa cell lysates were boiled in the presence of 0 mM or 140 mM 2-mercaptoethanol. Samples were separated by 8% SDS-PAGE, and analyzed by immunoblotting with GOLPH2 antibody. C and D, Secreted GOLPH2 from normal human urine (C) and HeLa cell supernatant (D) were prepared under non-reducing and reducing conditions (140 mM 2-mercaptoethanol). Immunoblotting revealed oligomer formation under non-reducing conditions.