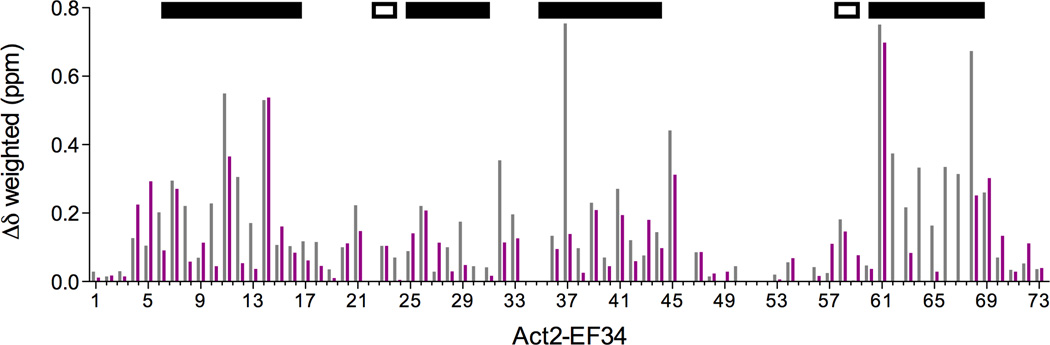
Figure 3.
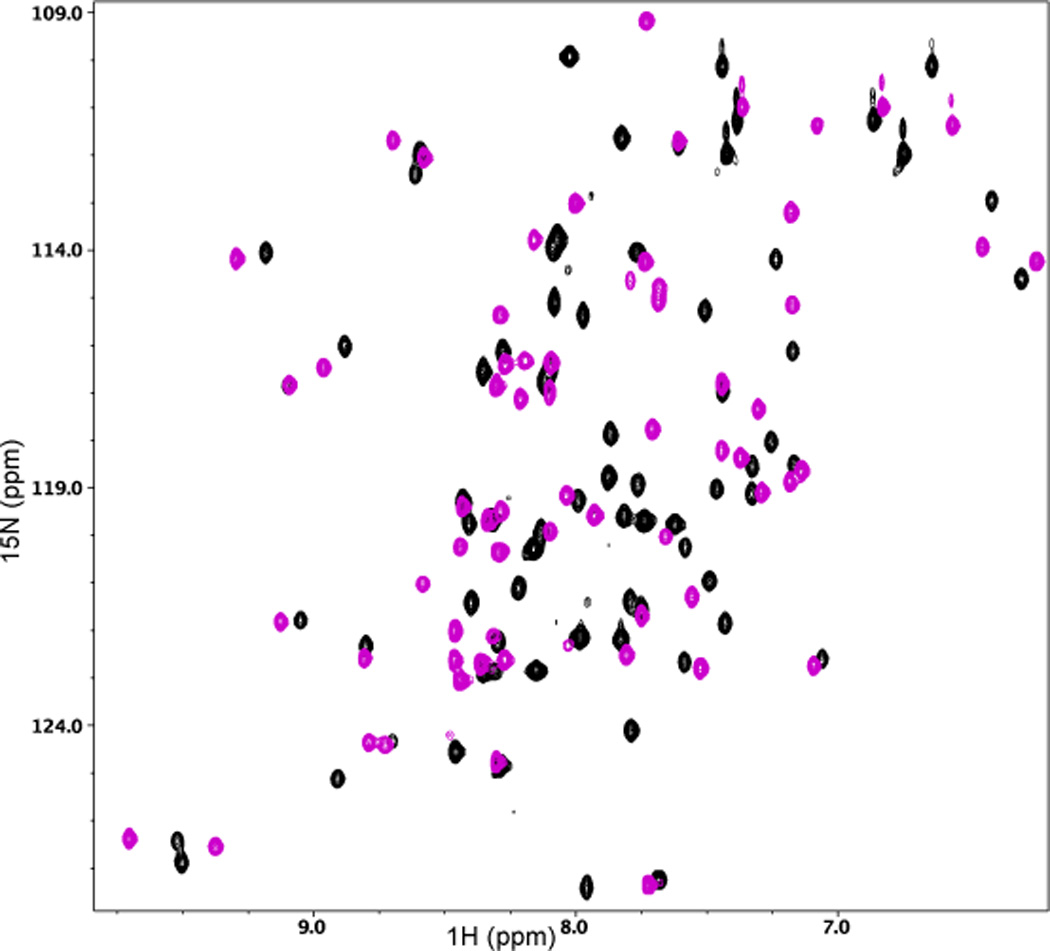
(a) 2D 1H-15N HSQC spectral overlay of 15N-labeled Act-EF34 in the absence (black) and presence of bound WT palladin peptide (magenta). (b) Chemical shift perturbation of Act-EF34 amide resonances upon complex formation with either titin Zr7 (gray) or palladin peptide (magenta). The α-helical regions or β-strands of Act-EF34 are designated by filled or open bars, respectively, above the plot. Also indicated (dotted line) is the chemical shift cut-off value used for the definition of AIRs as input for the HADDOCK calculations (0.17 ppm).