Table 2. Nickel-Catalyzed Benzylation of Ethylenea.
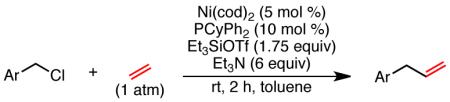 | ||
|---|---|---|
| para-substituted | ||
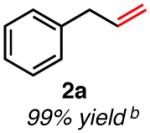
|
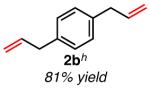
|
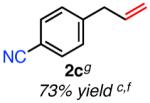
|
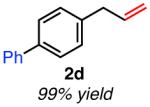
|
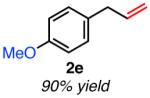
|
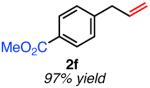
|
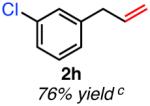
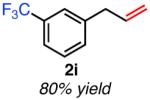
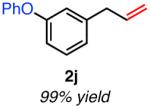
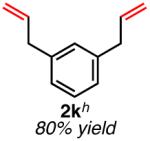
| meta-substituted | ||
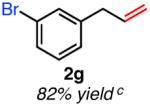
|
|
|
|
|
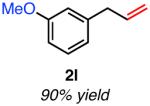
|
| hetero-substituted | ||
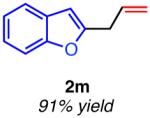
|
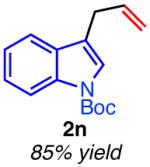
|
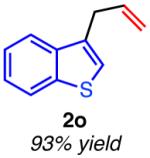
|
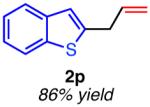
|
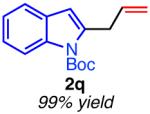
|
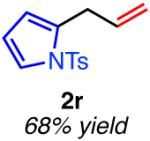
|
| ortho-substituted | ||
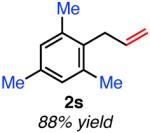
|
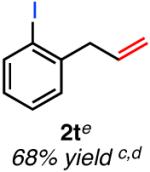
|
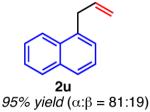
|
Isolated yield unless otherwise noted.
Determined by GC (internal standard).
Determined by 1H NMR spectroscopy (internal standard).
Yield obtained with 10 mol % catalyst.
2,3-Dihydro-1H-indene (4%) observed as inseparable byproduct.
Yield obtained with 20 mol % catalyst loading.
4-Cyano-β-methylstyrene (5%) obtained as an inseparable byproduct.
Obtained with 2.5 equiv Et3SiOTf.
