Figure 3. S-nitrosylation of Cys254 and Cys259 inhibits FLIP ubiquitination and degradation induced by FasL.
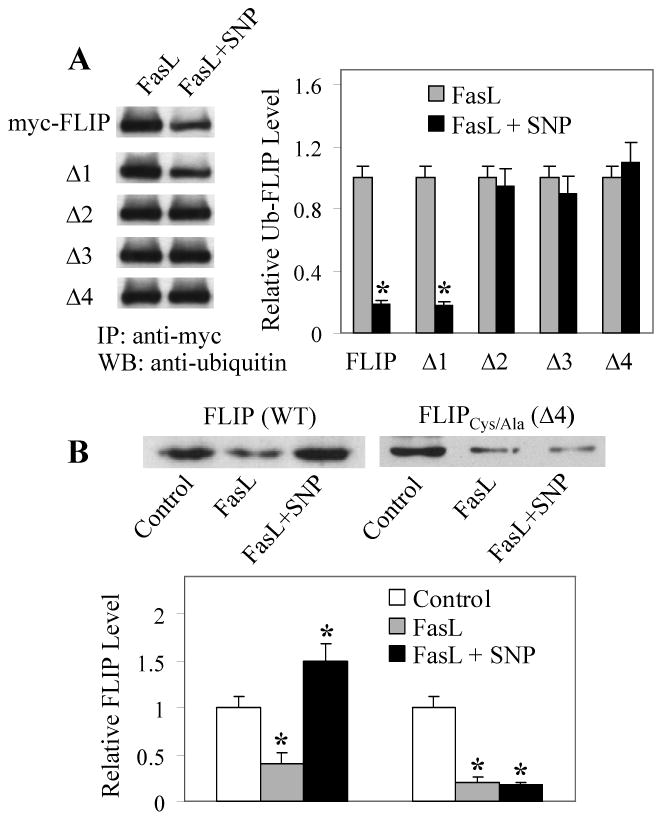
A, BEAS-2B cells were transiently transfected with ubiquitin and myc-FLIP or its mutant plasmids. One day after the transfection, cells were treated with FasL (100 ng/ml) in the presence or absence of SNP (300 μg/ml) for 2 h and cell lysates were prepared for immunoprecipitation using myc antibody. The immunoprecipitated proteins were analyzed by Western blot with antibody against ubiquitin. Band densities were normalized against FasL-treated controls. Plots are mean ± S.D. (n = 3). *p < 0.05 versus FasL-treated controls. B, Cells were transiently transfected with myc-FLIP or Δ4 mutant plasmid and then treated 1 day later with FasL (100 ng/ml) in the presence or absence of SNP (300 μg/ml) for 12 h. Cell lysates were immunoprecipitated with myc antibody and analyzed by Western blot using antibody against FLIP. Band densities were normalized against non-treated controls. Plots are mean ± S.D. (n = 3). *p < 0.05 versus non-treated controls. (Reproduced from ref. 22 with permission from the Journal of Biological Chemistry).
