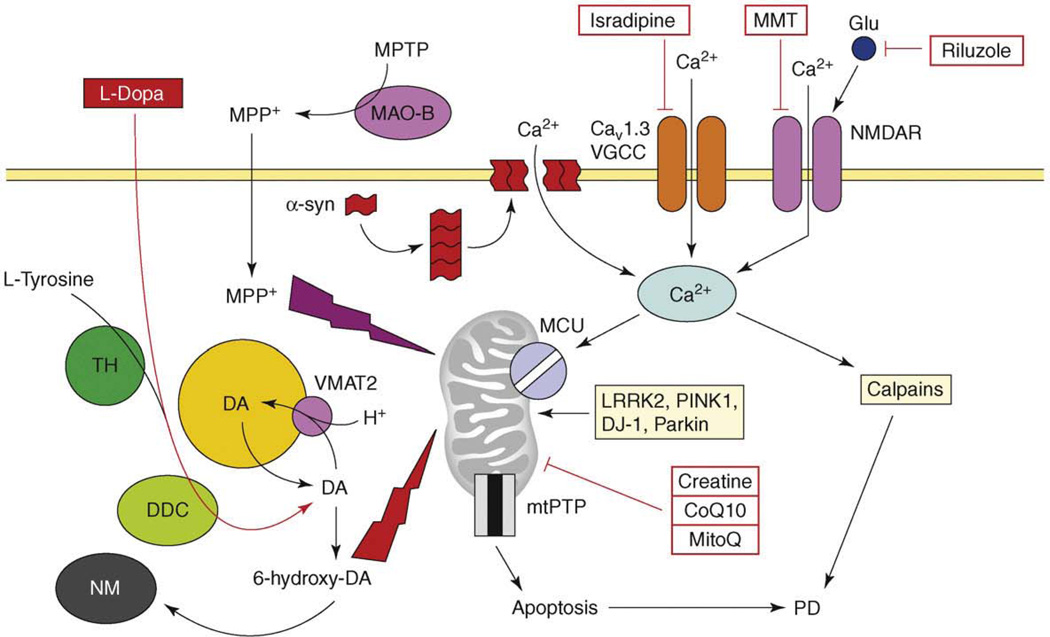
Figure 2.
The model of Ca2+ dysregulation in PD. Continuous Ca2+ influx to SNc neurons is mediated by CaV1.3 L-type voltage-gated Ca2+ channels (CaV1.3). In response to glutamate, Ca2+ influx is mediated by NMDA receptors (NMDAR). Alpha-synuclein forms aggregates (protofibrils) which may form Ca2+-permeable channels in the plasma membrane. Elevated cytosolic Ca2+ is transported into mitochondria through the activity of mitochondrial Ca2+ uniporter (MCU). Dopamine (DA) is generated from L-tyrosine by the action of tyrosine hydroxylase (TH) and is loaded into the synaptic vesicles by the activity of the DA/H+ cotransporter (VMAT2). Cytosolic DA is oxidized to 6-hydroxy-DA, which causes damage to proteins and mitochondria by oxidative stress. The products of DA oxidation accumulate as neuromelanin (NM). Cumulative damage to mitochondria resulting from Ca2+ overload and DA-mediated oxidative stress leads to an opening of mtPTP and apoptotic cell death of SNc neurons in PD. An importance of mitochondria is highlighted by several mitochondria-related genes (e.g. LRRK2, PINK1, DJ-1, Parkin), which are mutated in familial PD. In a chemical model of PD, the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is converted to the 1-methyl-4-phenylpyridinium (MPP+) by the glial enzyme monoamine oxidase B (MAO-B). MPP+ enters SNc neurons and potently inhibits mitochondrial complex I, causing selective cell death of SNc neurons. The US FDA-approved treatment for PD is levodopa (L-dopa), which is converted to DA by aromatic L-amino acid decarboxylase (DCC) inside SNc neurons. Generated DA is loaded to synaptic vesicles and alleviates symptoms of PD temporarily. The drugs tested or in PD clinical trials currently are ‘mitochondrial stabilizers’ (creatine, CoQ10, MitoQ), NMDAR antagonist memantine (MMT), antiglutamate agent riluzole and L-type VGCC inhibitor isradipine.