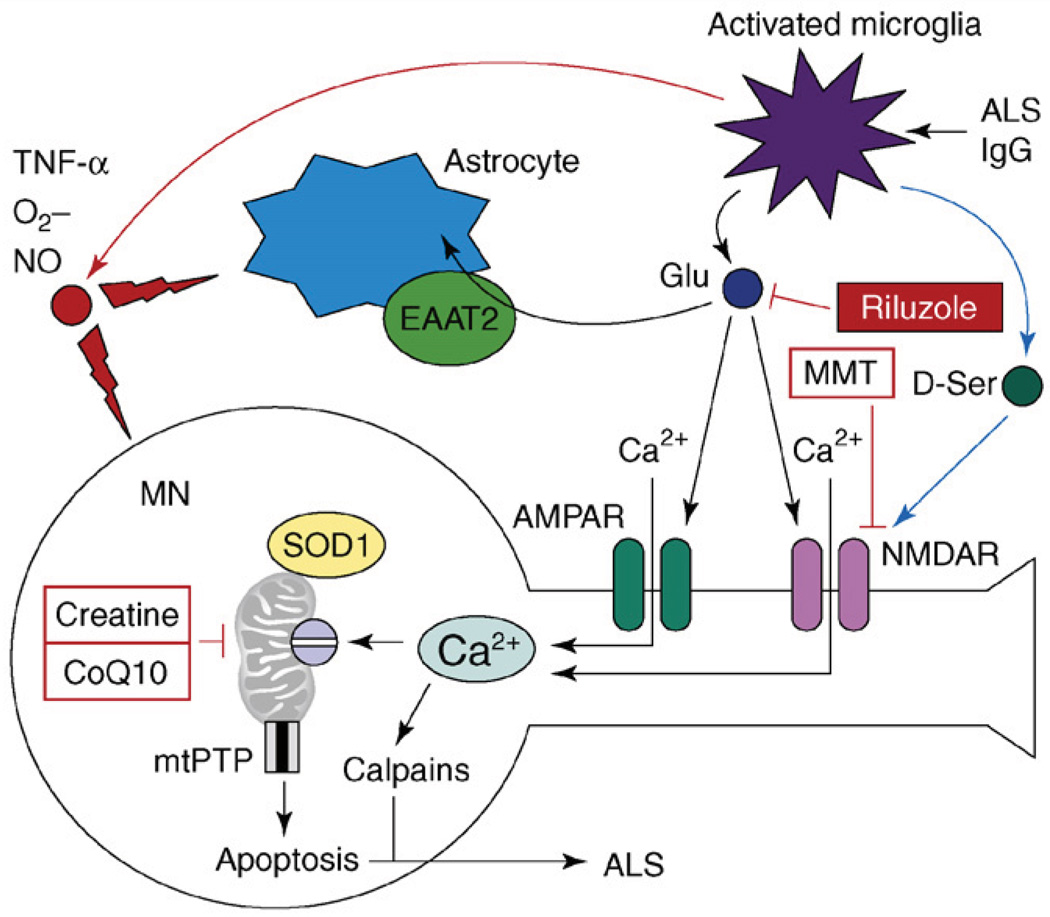
Figure 3.
The model of excitotoxicity and Ca2+ dysregulation in ALS. The pathogenic cascade in ALS involves interactions among activated microglia, astrocytes and motor neurons (MNs). In an experimental situation, microglia can be activated by antiserum collected from ALS patients (ALS IgG). Activated microglia release pro-inflammatory factors TNF-α, NO and O2−. Activated microglia also release large amounts of glutamate, which causes activation of AMPA and NMDA receptors on MNs. Activated microglia also release D-serine, which further sensitizes NMDAR to glutamate activation. Astrocytes express glutamate-uptake transporter EAAT2, which is involved in clearing glutamate from the extracellular space. Ca2+ influx by Ca2+-permeable AMPA receptors and NMDA receptors results in mitochondrial Ca2+ overload, mitochondrial swelling, opening of mitochondrial permeability-transition pore (mtPTP) and apoptosis of MNs. Mutant SOD1 binds to MN mitochondria and further impairs their ability to handle Ca2+ load. Antiglutamate agent riluzole is approved by the US FDA for treatment of ALS. NMDAR antagonist memantine (MMT) and ‘mitochondrial stabilizers’ creatine and CoQ10 are in ALS clinical trials currently.