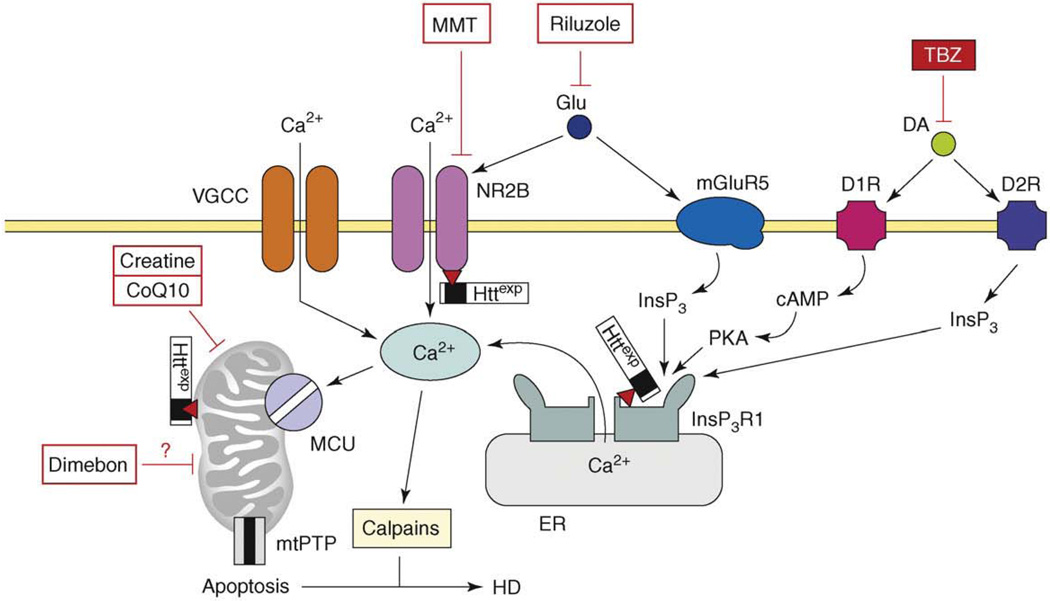
Figure 4.
The model of Ca2+ dysregulation in HD. In HD MSN, the Httexp perturbs Ca2+ signaling through several synergistic mechanisms. Httexp enhances function of NR2B-containing NMDAR, probably by promoting trafficking to the plasma membrane. Httexp binds strongly to the InsP3R1C terminus and sensitizes the InsP3R1 to activation by InsP3. The low levels of glutamate released from corticostriatal projection neurons lead to supranormal Ca2+ influx by NMDAR and Ca2+ release through the InsP3R1. Additional Ca2+ influx to MSN is mediated by voltage-gated Ca2+ channels (VGCCs). Dopamine released from midbrain dopaminergic neurons stimulates D1-class and D2-class DARs, which are expressed abundantly in MSNs. D1-class DARs are coupled to activation of adenyl cyclase, increase in cAMP levels and activation of PKA. PKA potentiates glutamate-induced Ca2+ signals by facilitating the activity of NMDAR and InsP3R1. D2 receptors are coupled directly to InsP3 production and activation of InsP3R1. Supranormal Ca2+ signals activate calpain, which cleave Httexp and other substrates. Excessive cytosolic Ca2+ signals result in mitochondrial Ca2+ uptake by MCU, which eventually triggers mtPTP opening and apoptosis. The mitochondrial Ca2+ handling is further destabilized by direct association of Httexp with mitochondria. Antidopamine agent tetrabenazine (TBZ) is approved by the US FDA for symptomatic treatment of HD. NMDAR antagonist memantine (MMT), putative ‘mitochondrial agent’ Dimebon and ‘mitochondrial stabilizers’ creatine and CoQ10 are tested in HD clinical trials. Antiglutamate agent riluzole was tested and failed [66]. Adapted from [77].