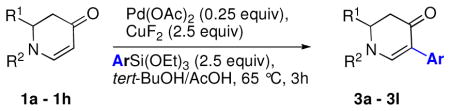
Table 2.
Substrate Scope of Hiyama Coupling Reactions
 | |||
|---|---|---|---|
| entrya | enaminone | product | yield (%)b |
| 1 |
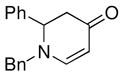 1a |
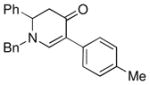 3b |
85 |
| 2 |
 1a |
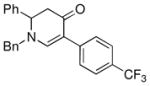 3c |
73 |
| 3 |
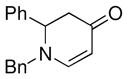 1a |
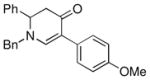 3d |
50 |
| 4 |
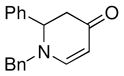 1a |
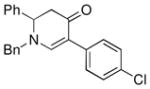 3e |
65 |
| 5 |
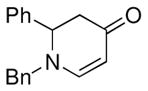 1a |
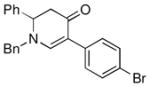 3f |
61 |
| 6c |
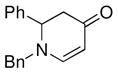 1a |
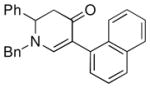 3g |
43 |
| 7 |
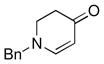 1b |
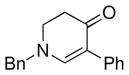 3h |
62 |
| 8 |
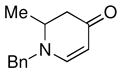 1c |
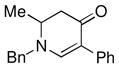 3i |
81 |
| 9 |
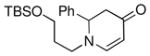 1d |
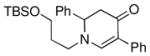 3j |
75 |
| 10 |
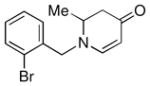 1e |
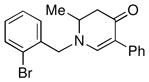 3k |
68 |
| 11 |
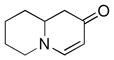 1f |
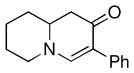 3l |
72 |
| 12d |
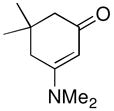 1g |
not observed | 0 |
| 13d |
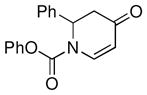 1h |
not observed | 0 |
Reaction conditions: enaminone (0.1 M), Pd(OAc)2 (0.25 equiv), CuF2 (2.5 equiv), ArSi(OEt)3 (2.5 equiv) in tBuOH/AcOH (4:1), 65 °C, 3h.
Isolated yield.
α-Naphthyltrimethoxysilane was used.
Starting material recovered.
