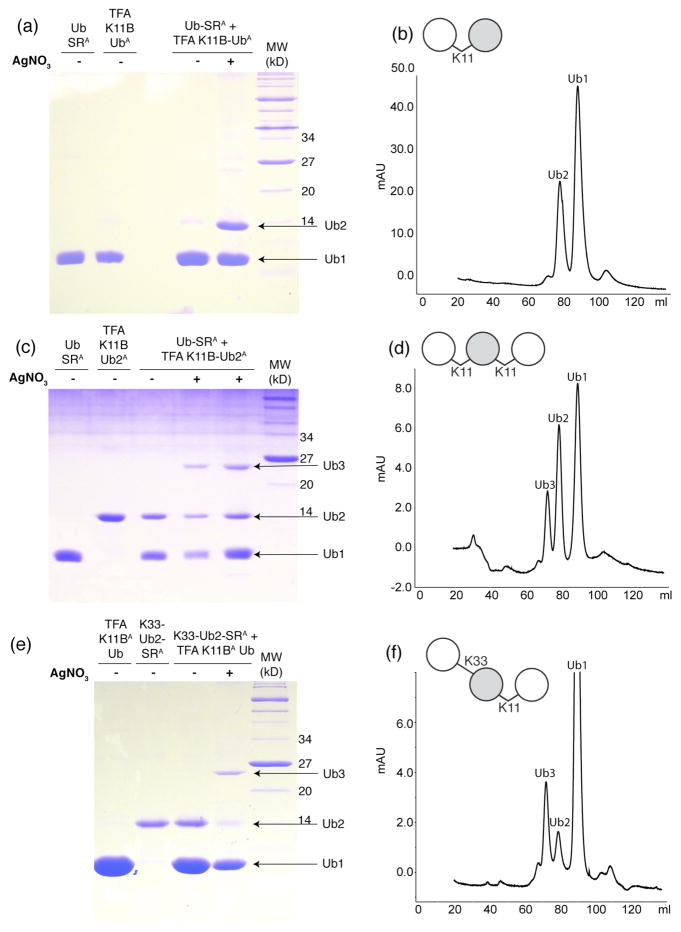
Figure 2.
Chemical assembly of K11-linked di- and tri-Ub chains. (a,c,e) Coomassie-stained 15% SDS PAGE gels of the chemical condensation reactions of (a) K11-linked Ub2 15N-labeled on the proximal Ub, (c) K11-linked Ub3 with the middle Ub unit 15N-labeled, and (e) K33,K11-linked Ub3 with the middle Ub unit 15N-labeled. K11-linked Ub2 (panel a) was assembled from Alloc-protected monomers of Ub-SR (Ub-SRA) and TFA-treated 15N-labeled K11Boc Ub (TFA K11B UbA). K11-linked Ub3 (panel c) was assembled from Alloc-protected Ub-SR and distal-15N-labeled K11-linked Ub2 (TFA K11B Ub2A) whose distal-K11 side chain was made available for ligation by TFA treatment. K33,K11-linked Ub3 (panel e) was assembled from Alloc-protected proximal-15N- labeled K33-linked Ub2 (K33-Ub2-SR) and TFA-treated K11Boc Ub (TFA K11B UbA). Formation of Ub2 or Ub3 is observed after 20 hours of reacting the proteins with AgNO3, H-OSu, and DIEA. 0.3 – 0.4 μL are loaded from the reaction directly onto gel. Notations used here: “TFA” indicates that the corresponding protein was treated with TFA to remove the Boc group in order to make the corresponding Lys available for the ligation reaction, while the superscript “A” indicates that the protein is Alloc-protected.
(b,d,f) Size-exclusion chromatograms of (b) K11-linked Ub2, (d) homogeneously K11-linked Ub3, and (f) mixed-linkage K33,K11-linked Ub3 performed after Alloc deprotection and protein renaturation to purify the desired product from the unreacted components. The ability to separate Ub2 or Ub3 products from unreacted species is illustrated in Fig. S8.