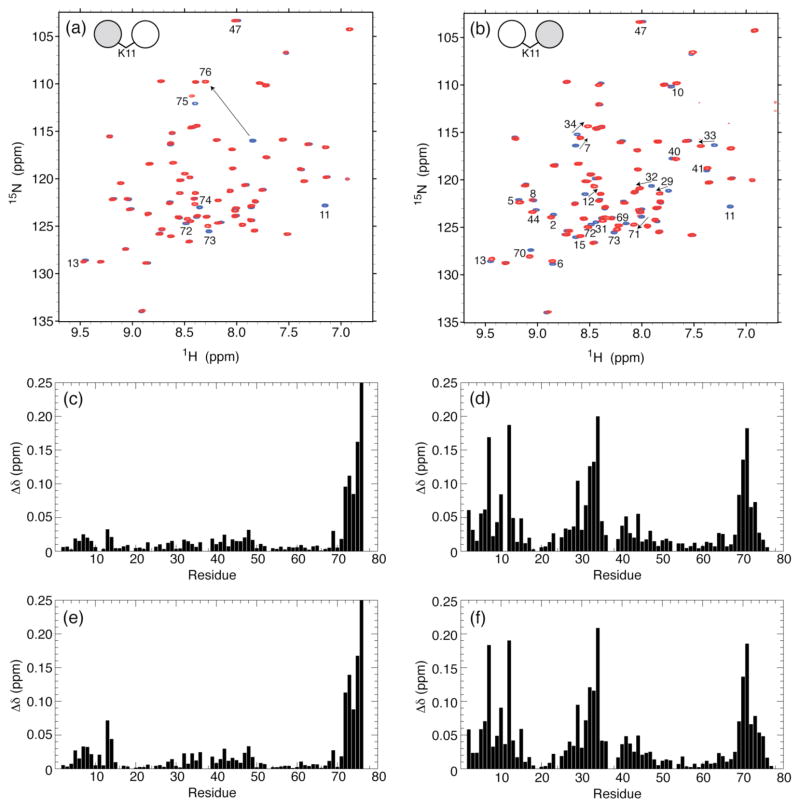
Figure 4.
Overlay of 1H-15N TROSY-HSQC spectra of (a) the distal Ub and (b) the proximal Ub in all-natural K11-linked Ub2 (red) and of WT Ub (blue). The spectral differences between K11-linked di-Ub and mono-Ub, quantified as amide chemical shift perturbations (CSPs), are plotted as a function of the residue number for (c,e) distal Ub and (d,f) proximal Ub in (c,d) all-natural K11-linked Ub2 and (e,f) enzymatically-synthesized K11-linked Ub2. The CSPs were calculated as Δδ = [(ΔδH)2 + (ΔδN/5)2]1/2, where ΔδH and ΔδN are chemical shift differences for 1H and 15N, respectively. Residues with significant CSPs are indicated on the spectra in panels a and b. Note that the absence of the K11 backbone amide signal in the spectra of K11-linked Ub2 (panels a and b) serves as a direct confirmation of the incorporation of the unlabeled Lys at residue 11.