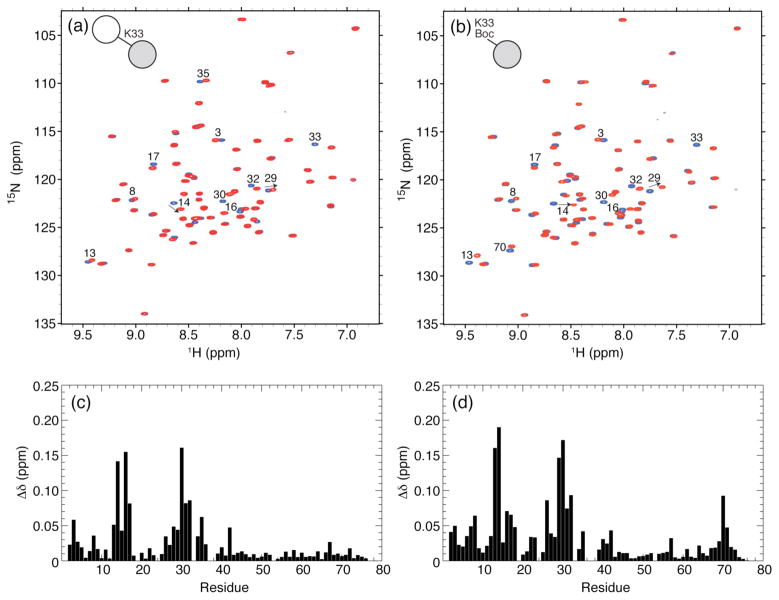
Figure 5.
(a) Overlay of 1H-15N TROSY spectra of the proximal Ub in K33-linked Ub2 (red) and WT monoUb (blue). (b) Overlay of 1H-15N TROSY spectra of K33Boc Ub (red) and WT Ub (blue). (c,d) The spectral differences between residues in the (c) proximal Ub of K33-linked Ub2 and WT Ub and in (d) K33Boc Ub and WT Ub are quantified as amide chemical shift perturbations (CSPs). Residues with significant CSPs are indicated on the spectra in panels a and b. The absence of the K33 backbone amide signal in the spectra of K33-linked Ub2 and K33Boc Ub (panels a and b, respectively) serves as a direct confirmation of the incorporation of the unlabeled Lys at residue 33.