Abstract
Background
Nocturia, a common complaint in aging men and women, is frequently cited as the cause of nocturnal awakenings leading to sleep loss, daytime fatigue, and reduced quality of life (QOL).
Objective
Investigate the association of nocturia with QOL and depressive symptoms among men and women.
Design, setting, and participants
A population-based epidemiologic survey of urologic symptoms among persons aged 30–79 yr. A multistage stratified cluster sample design was used to randomly sample 5503 residents of Boston, MA, USA.
Measurements
Nocturia was defined as a self-report of two or more voiding episodes nightly or having to get up to urinate more than once nightly “fairly often,” “usually,” or “almost always.” QOL was assessed using the physical and mental health component scores of the 12-Item Short-Form Survey (SF-12). Depression was assessed using the Center for Epidemiological Studies Depression Scale. Multiple linear and logistic regression methods were used to model the nocturia and QOL association and to control for confounders.
Results and limitations
Nocturia was associated with decreased SF-12 scores for both the physical and mental health components after multivariate adjustment. Nocturia was also associated with increased odds of depressive symptoms (men: adjusted odds ratio [OR]: 2.79; 95% confidence interval [CI], 1.81–4.31; women: adjusted OR: 1.80; 95% CI, 1.29–2.51). Among women who reported sleep interference due to urologic symptoms, nocturia was associated with a threefold increase in odds of depression. In this cross-sectional analysis, the temporal sequence of causality of the nocturia and depression association could not be assessed.
Conclusions
Nocturia is associated with decreased QOL and with an increased prevalence of depressive symptoms in both men and women.
Keywords: Nocturia, Quality of life, Depression, Epidemiology
1. Introduction
Nocturia (nighttime voiding) is a common and bothersome urologic symptom [1–3] and is the most frequently cited cause of nocturnal awakenings resulting in sleep loss, daytime fatigue, and mood disturbance [1,4–6]. Previous studies have reported symptom bother and reduced quality of life (QOL) associated with nocturia [1,7]. However, data are inconsistent on frequency of nocturnal voiding and the level at which the frequency significantly affects QOL and bother. One study reported adverse effects with only one voiding episode nightly [1], while another showed significant effects with two or more nightly episodes [7]. Studies have consistently shown an increase in depression with nocturia, but they were conducted among men and primarily in older age groups [8–10].
Previous results from the Boston Area Community Health (BACH) Survey show decreased overall QOL with increasing severity of lower urinary tract symptoms as assessed by the International Prostate Symptom Score (IPSS) [11]. Current objectives are (1) to determine the effect of nocturia on QOL and symptom bother, (2) to investigate the association of nocturia with depression, and (3) to determine whether the association of number of nightly voids with QOL and depression follows a dose-response pattern.
2. Methods
2.1. Overall design
BACH is a population-based epidemiologic survey of urologic symptoms and risk factors. Detailed methods have been described elsewhere [12]. Eligibility criteria include the ability to speak English or Spanish and being cognitively able to provide informed consent. A multistage stratified design was used to randomly recruit approximately equal numbers according to age (30–39, 40–49, 50–59, and 60–79 yr), gender, and race and ethnic group (black, Hispanic, and white). Cross-sectional data from the baseline assessment (April 2002–June 2005) were used for this analysis. Interviews were completed with 5503 subjects (2301 men, 3202 women), or 63.3% of 8702 eligible subjects. All protocols and informed consent procedures were approved by the New England Research Institute’s institutional review board.
2.2. Data collection
Data were obtained during a 2-h in-person interview conducted by a trained interviewer, generally in the subject’s home. Pregnant women and women who had given birth within the last 6 mo were rescheduled to have study visits at least 6 mo after delivery. Height, weight, and hip and waist circumference were measured. Self-reported information on major comorbidities, lifestyle and psychosocial factors, and symptoms of urogynecologic conditions was collected. Medication use in the past month was collected using a combination of drug inventory and self-report with a prompt by indication.
2.3. Nocturia
Nocturia was defined in two ways to provide more robust analyses. The first definition used two questions: (1) “During last month how often have you had to get up to urinate more than once during the night?” and (2) “In the last 7 days on average how many times have you had to go to the bathroom to empty your bladder during the night after falling asleep?” If the response to question 1 was “fairly often,” “usually,” or “almost always,” or the response to question 2 was two or more, nocturia was considered present. This broad definition of nocturia has been used in previous BACH study reports [13,14]. The second definition was based on the number of self-reported nightly voids (0, 1, 2, ≥3) to allow examination of trends.
2.4. Quality-of-life measures
Overall QOL was assessed using the 12-Item Short-Form Survey (SF-12), which includes both the physical component score (PCS-12) and the mental health component score (MCS-12) [15]. These scores are standardized to have a mean (standard deviation) of 50 (10) in the general US population (higher scores indicate better QOL) and to approximate a normal distribution. Interference with activities of daily living by urologic symptoms was assessed using the validated Epstein scale; higher scores indicate greater interference [16]. The total score was obtained by summing individual scores from seven questions on interference of urinary symptoms with various activities (0 for “none of the time” to 4 for “all of the time”). These scores show a positive skew. The frequency of the sleep interference item on the Epstein scale was used to assess sleep disturbance. The presence of depressive symptoms was assessed using the abbreviated Center for Epidemiological Studies Depression Scale. Participants reporting five or more depressive symptoms (out of eight) were considered to have clinically significant depression [17].
2.5. Covariates
Covariates included age; self-reported race/ethnicity; body mass index (BMI) categorized as <25, 25–29, or ≥30 kg/m2; physical activity assessed using the Physical Activity Scale for the Elderly and categorized as low (<100), medium (100–250), or high (>250) [18]; alcohol consumption categorized as alcoholic drinks consumed per day (0, <1, 1–3, or >3); smoking as never, former, or current; and marital status as married, living with a partner, divorced/separated, widowed, or single. The socioeconomic status (SES) index was calculated using a combination of education and household income [19] and was categorized as low (lower 25% of the distribution), middle (middle 50%), or high (upper 25%). Comorbidities were defined as a yes response to “Have you ever been told by a health care provider that you have or had … ?” and included heart disease, hypertension, diabetes, and arthritis. Heart disease included self-report of myocardial infarction, angina, congestive heart failure, coronary artery bypass, or angioplasty stent. Prescription medications were grouped as follows: (1) medications for overactive bladder/urinary incontinence (oxybutynin, tolterodine tartrate, propantheline, hyoscyamine sulfate), (2) medications for painful bladder syndrome (pentosan polysulfate sodium, amitriptyline, imipramine, hydroxyzine), (3) medications for benign prostatic hyperplasia (doxazosin, terazosin, prazosin, alfuzosin, tamsulosin, finasteride), (4) diuretics of any class, and (5) selective serotonin reuptake inhibitors(SSRIs)/serotonin–norepinephrine reuptake inhibitors (SNRIs)/serotonin modulator antidepressants (ADs).
2.6. Statistical analysis
Analyses were conducted separately by sex. Multivariate linear regression was used to model the association of nocturia with continuous SF-12 and interference (Epstein) scores. Confounder-adjusted means and 95% confidence intervals (CIs) are reported. Confounder-adjusted odds ratios (ORs) and 95% CI were estimated using multivariate logistic regression to assess the association of nocturia and depression. Aside from age and race/ethnicity (always included as design variables), additional covariates were included in logistic regression models if their overall significance was p < 0.05 or if they changed the OR by >10%. Multiple imputation was used to obtain plausible values for missing data [17].The proportion with missing data was 0.4% (n = 22) for nocturia, 0.9% (n = 47) for comorbid conditions and depression, and 0.9% (n = 49) for lifestyle variables (physical activity, alcohol consumption, and smoking). The SES index was missing for 6.1% of participants (n = 333) primarily because of missing data on household income rather than years of education (0.3%, n = 16). Overall, 7.7% of BACH participants had missing data on at least one of these variables. To be representative of the city of Boston (MA, USA), observations were weighted inversely proportional to their probability of selection [20]. Weights were poststratified to the Boston population according to the 2000 census. Analyses were conducted in SAS 9.1 (SAS Institutes, Cary, NC, USA) and SUDAAN 9.01 (Research Triangle Institutes, Research Triangle Park, NC, USA).
3. Results
Analytic sample characteristics are presented in Table 1. Prevalence of nocturia was 25.3% in men and 31.3% in women, and the prevalence increased to approximately 40% in men and women aged ≥60 yr.
Table 1.
Descriptive characteristics of analysis sample
| Characteristic | Men | Women |
|---|---|---|
| Age | ||
|
| ||
| Mean (SD) | 47.6 (12.8) | 49.2 (13.3) |
| 30–39, n (%) | 615 (37.2) | 792 (33.4) |
| 40–49, n (%) | 659 (25.8) | 839 (24.4) |
| 50–59, n (%) | 510 (17.8) | 777 (18.4) |
| 60–79, n (%) | 517 (19.2) | 794 (23.7) |
| Race/ethnicity, n (%) | ||
| White | 835 (36.3*) | 1024 (32.0*) |
| Black | 700 (30.4*) | 1067 (33.3*) |
| Hispanic | 766 (33.3*) | 1111 (34.7*) |
| Socioeconomic status, n (%) | ||
| Low | 970 (24.3) | 1595 (30.8) |
| Middle | 954 (49.1) | 1199 (45.2) |
| High | 377 (26.6) | 408 (23.9) |
| Marital status, n (%) | ||
| Married | 1075 (46.3) | 972 (36.1) |
| Living with a partner | 194 (9.0) | 248 (8.9) |
| Divorced/separated | 354 (12.1) | 796 (18.7) |
| Widowed | 64 (2.6) | 345 (9.3) |
| Single, never married | 614 (30.0) | 842 (27.0) |
| Body mass index, kg/m2, n (%) | ||
| <25 | 595 (26.6) | 753 (33.3) |
| 25–29 | 905 (40.7) | 972 (28.6) |
| ≥30 | 801 (32.7) | 1477 (38.1) |
| Physical activity (Physical Activity Scale for the Elderly), n (%) |
||
| Low (<100) | 694 (26.8) | 1191 (27.8) |
| Moderate (200–250) | 1068 (47.4) | 1576 (53.6) |
| High (>250) | 538 (25.8) | 434 (18.5) |
| Cigarette smoking, n (%) | ||
| Never | 964 (45.1) | 1704 (50.2) |
| Former | 662 (28.7) | 776 (27.2) |
| Current | 675 (26.2) | 722 (22.6) |
| Alcohol consumption (drinks per day), n (%) |
||
| None | 800 (27.5) | 1661 (41.7) |
| <1 | 815 (38.9) | 1209 (43.2) |
| 1–2.9 | 434 (24.1) | 268 (12.9) |
| ≥3 | 252 (9.6) | 64 (2.2) |
| Heart disease, n (%) | 248 (10.1) | 303 (7.9) |
| Hypertension, n (%) | 735 (26.1) | 1125 (28.3) |
| Diabetes, n (%) | 298 (9.3) | 449 (9.6) |
| Arthritis, n (%) | 444 (17.3) | 1063 (29.0) |
| Depression (CESD ≥5), n (%) | 391 (14.0) | 830 (20.1) |
| Prescription medication use, n (%) | ||
| For UI, OAB | 16 (0.5) | 53 (1.7) |
| For painful bladder | 21 (0.8) | 75 (1.7) |
| For BPH | 110 (3.5) | 0 (0.0) |
| Diuretics | 256 (8.1) | 564 (13.8) |
| Antidepressants | 227 (11.9) | 521 (17.1) |
| SF-12 scores | ||
| PCS-12, mean (SD) | 50.3 (9.3) | 48.2 (10.8) |
| MCS-12, mean (SD) | 50.4 (9.8) | 49.2 (10.6) |
| Interference score (Epstein scale) | ||
| Mean (SD) | 1.3 (3.6) | 2.2 (4.8) |
| Median (interquartile range) | 0 (0–2) | 0 (0–2) |
| Interference getting enough sleep, n (%) |
313 (12.4) | 663 (18.1) |
CESD = Center for Epidemiological Studies Depression Scale; UI = urinary incontinence; OAB = overactive bladder; BPH = benign prostatic hyperplasia; SF-12 = 12-Item Short-Form Survey; PCS-12 = physical component score of SF-12; MCS-12 = mental health component score of SF-12; SD = standard deviation.
Unweighted proportions.
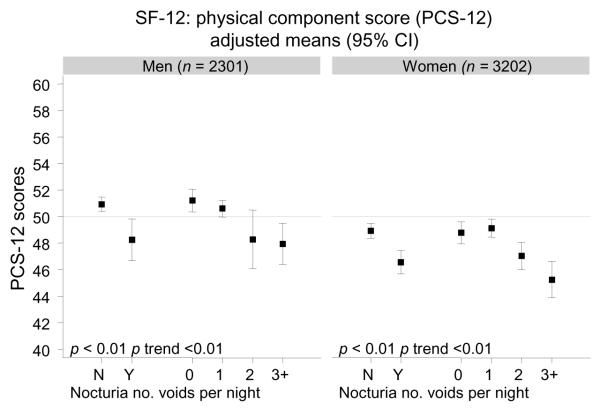
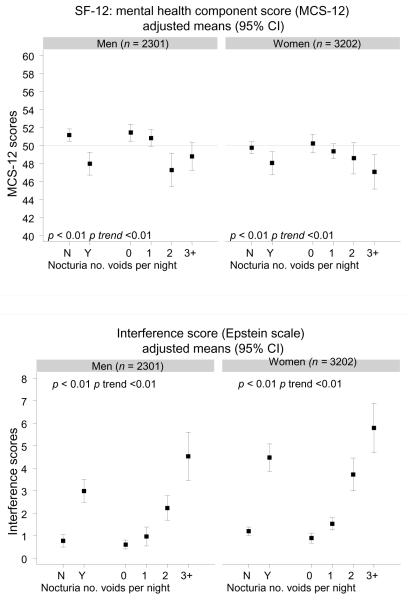
A decrease in both mean physical and mean mental health component scores was observed by nocturia and number of nightly voids after accounting for potential confounders (Fig. 1). A significant trend of decreasing SF-12 scores with increasing number of nightly voids was observed. The largest drop in mean PCS-12 scores was observed between one and two voids nightly. A similar trend was observed for mean MCS-12 scores in men, while the decrease in MCS-12 scores in women appeared more linear. An increase in mean interference scores on the Epstein scale was observed for nocturia, with significant trends of higher scores with more nightly voids for both genders.
Fig. 1.
Quality-of-life measures (12-Item Short-Form Survey [SF-12], interference score) by nocturia and number of voids per night in men and women; mean SF-12 (physical component score [PCS-12] and mental health component score [MCS-12]) and interference (Epstein scale) scores adjusted for age, race/ethnicity, socioeconomic status, body mass index, physical activity, smoking, alcohol consumption, heart disease, hypertension, diabetes, and arthritis
CI = confidence interval; N = no; Y = yes.
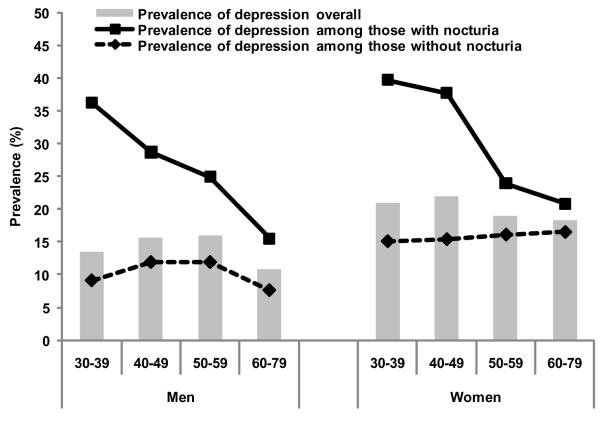
The overall prevalence of depression was 14.0% in men and 20.1% in women and showed little variation with age (Fig. 2). A higher prevalence of depression with nocturia was observed in younger groups; however, this difference decreased with age. Nocturia was associated with increased odds of depression with statistically significant trends with higher number of voids (Table 2). Analyses stratified by age, AD use, and sleep interference are presented in Table 3. Among men, an association of nocturia and depression was observed at ages <65 yr, but there was no significant effect modification by age (p value >0.10). Among women, an association of nocturia and depression was observed only in ages <50 yr. When stratifying by sleep interference, a threefold increase in the odds of depression was observed with nocturia among women who reported sleep interference due to urologic symptoms (adjusted OR: 3.37; 95% CI, 1.63–6.94), while no association was observed among women who reported no sleep interference (adjusted OR: 1.10; 95% CI, 0.74–1.54). The difference by sleep interference in men was smaller and statistically nonsignificant. No effect modification of the nocturia and depression association was observed for AD use or for the other covariates included in the analysis. Of additional urologic symptoms assessed using the IPSS, frequency was reported most commonly in 33.4% of men and 42.4% of women with nocturia, while overlap of nocturia with urgency, incomplete emptying, weak stream, straining, and intermittency ranged between 6.9% and 19.1%. Further adjustment for these urologic symptoms, urinary tract infections in the past 12 mo, history of renal disease, marital status, erectile dysfunction in men (assessed using the Five-Item International Index of Erectile Function), or use of waist circumference or waist-to-hip ratio instead of BMI did not alter the results (data not shown).
Fig. 2. Prevalence of depression (Center for Epidemiological Studies Depression Scale ≥5) by gender, age, and nocturia status.
Take-home message
Nocturia is associated with increased symptom bother and depressive symptoms. Two or more nighttime voiding episodes constitute a threshold level for nocturia beyond which adverse effects on quality of life are detected.
Table 2.
Association of nocturia and depression (Center for Epidemiological Studies Depression Scale ≥5): odds ratios and 95% confidence intervals
| Age and race/ethnicity |
Multivariate | Model 1 | |
|---|---|---|---|
| Nocturia: no. voids per night |
Adjusted, odds ratio (95% CI) |
Model 1,* odds ratio (95% CI) |
Plus sleep interference, odds ratio (95% CI) |
| Men | 3.33 (2.06–5.39) | 2.37 (1.59–3.55) | 2.01 (1.27–3.17) |
| 0 | 1.00 | 1.00 | 1.00 |
| 1 | 1.17 (0.72–1.88) | 1.05 (0.64–1.71) | 1.03 (0.63–1.68) |
| 2 | 3.99 (1.96–8.11) | 2.46 (1.36–4.45) | 2.08 (1.11–3.92) |
| 3+ | 3.36 (1.88–6.01) | 2.68 (1.45–4.93) | 2.24 (1.15–4.34) |
| p trend | <0.001 | <0.001 | 0.007 |
| Women | 2.18 (1.55–3.05) | 1.77 (1.27–2.48) | 1.40 (1.02–1.94) |
| 0 | 1.00 | 1.00 | 1.00 |
| 1 | 1.38 (0.92–2.07) | 1.18 (0.78–1.80) | 1.13 (0.73–1.73) |
| 2 | 1.85 (1.15–2.98) | 1.51 (0.93–2.44) | 1.22 (0.75–1.98) |
| 3+ | 3.79 (2.36–6.10) | 2.63 (1.60–4.32) | 1.94 (1.21–3.13) |
| p trend | <0.001 | <0.001 | 0.010 |
CI = confidence interval.
Model for men adjusted for age, race/ethnicity, socioeconomic status, physical activity, smoking, alcohol consumption, arthritis, and antidepressant use. Model for women adjusted for age, race/ethnicity, socioeconomic status, smoking, alcohol consumption, heart disease, diabetes, and antidepressant use.
Table 3.
Association of nocturia and depression (Center for Epidemiological Studies Depression Scale ≥5) stratified by age, antidepressant use, and sleep interference due to urinary symptoms: odds ratios and 95% confidence intervals
| Men |
Women |
|||
|---|---|---|---|---|
| Characteristic | Unadjusted, odds ratio (95% CI) |
Multivariate,* odds ratio (95% CI) |
Unadjusted, odds ratio (95% CI) |
Multivariate,** odds ratio (95% CI) |
| Age, yr | ||||
|
| ||||
| 30–49 | 4.26 (2.33–7.81) | 3.25 (1.81–5.84) | 3.53 (2.26– 5.54) |
2.34 (1.46– 3.75) |
| 50–64 | 2.25 (1.30–3.91) | 2.45 (1.31–4.57) | 1.38 (0.85– 2.25) |
1.15 (0.69– 1.89) |
| ≥65 | 2.53 (0.99–6.47) | 1.48 (0.69–3.16) | 1.72 (0.90– 3.31) |
1.48 (0.68– 3.23) |
| p interaction | 0.266 | 0.630 | 0.013 | 0.030 |
| SSRI/SNRI use |
||||
| No | 2.54 (1.72–3.76) | 2.10 (1.33–3.33) | 2.00 (1.42– 2.81) |
1.59 (1.09– 2.33) |
| Yes | 3.47 (1.43–8.41) | 2.66 (1.15–6.16) | 3.40 (1.79– 6.45) |
2.52 (1.31– 4.84) |
| p interaction | 0.516 | 0.830 | 0.131 | 0.175 |
| Sleep interference |
||||
| No | 2.82 (1.63–4.88) | 2.43 (1.41–4.20) | 1.40 (0.95– 2.07) |
1.10 (0.74– 1.64) |
| Yes | 1.84 (0.76–4.46) | 2.18 (0.73–6.53) | 4.15 (2.25– 7.67) |
3.37 (1.63– 6.94) |
| p interaction | 0.428 | 0.635 | 0.002 | 0.009 |
CI = confidence interval; SSRI/SNRI = selective serotonin reuptake inhibitor/serotonin–norepinephrine reuptake inhibitor.
Adjusted for age, race/ethnicity, socioeconomic status, physical activity, smoking, alcohol consumption, and arthritis.
Adjusted for age race/ethnicity, socioeconomic status, smoking, alcohol consumption, heart disease, and diabetes.
4. Discussion
The results of the BACH study demonstrate a significant association of nocturia with decreased QOL and interference with daily activities in both men and women. Nocturia was also associated with increased likelihood of depression, especially among younger men and women. An adverse effect of nocturia on QOL has been reported previously. A nested case-control study of overactive bladder and QOL showed that nocturia was associated with a decrease in the physical function, vitality, and mental health function subscales of the SF-36 measures of QOL, as well as with decreased hours and adequacy of sleep [1]. These results suggest that even one voiding episode nightly is associated with increased bother, reduced sleep, and decreased QOL. Similar associations were reported in a Finnish population-based study [7]. In contrast with the previous study, an effect of nocturia on bother and QOL was observed only with two or more voiding episodes nightly. Results from the BACH study are consistent with these findings, with the largest decrease in SF-12 scores observed among subjects with two or more voids nightly among both genders. The observed changes in SF-12 scores (in the range of 3–5 points) are in the range of minimal important differences, the smallest differences that are perceived as important to the subject, for this scale [21]
Consistent with previous reports of higher prevalence of nocturia in elderly men with depression [8,10], our results show a strong association of nocturia with depression in both men and women, with a significant trend in increased odds of depression with more voids nightly. The magnitude of this association was larger in younger age groups, especially among women <50 yr. Some studies have suggested that urinary symptoms are accepted as part of aging, which may result in less perceived bother among older adults [22,23]. Our results also suggest that the nocturia and depression association may be mediated by sleep interference in women but not in men. Both depression and sleep disturbances are more commonly reported in women. Additionally, women have been reported to have a higher level of bother with nocturia than men [6]. Recent studies suggest sleep disturbance and nocturia to affect QOL independently among Chinese men and women [24] and report an association of nocturia and sleep disturbance in men with moderate or severe lower urinary tract symptoms [25]. Although our results suggest that nocturia may lead, partly through sleep disturbance, to the development of depression, the temporal sequence of causality of the association cannot be determined from the present cross-sectional analyses. Adverse health outcomes of reduced sleep duration and quality, including increased risk of obesity and diabetes and reduced cognitive and physical function, are well established [26–30]. Results from the Tampere Aging Male Urologic Study, a population-based longitudinal study of men 50–79 yr, showed no association between nocturia at baseline and depression at follow-up. However, untreated baseline depression was associated with incident nocturia [9]. As these are the only data addressing the temporal sequence of nocturia and depression, additional longitudinal studies are needed.
Potential limitations of this study should be noted. Although history of comorbid conditions was assessed by self-report, with the potential for associated biases, previous research has demonstrated the reliability and validity of self-report for heart disease, diabetes, and hypertension [31–33]. Observed associations could be further affected by disease duration and treatment history, which were not considered in this analysis. Additionally, BACH did not include a neurologic assessment or data on life events, sleep apnea, and other sleep-related conditions. Study strengths include a community-based random sample of a broad age range, inclusion of both genders, representation for black and Hispanic populations, and a wide range of covariates. The BACH study was limited geographically to the Boston area. However, comparison of sociodemographic and health-related variables from BACH with other large regional surveys (Boston Behavioral Risk Factor Surveillance System) and national surveys (National Health Interview Survey) has shown that BACH estimates are comparable to national trends on key health-related variables.
5. Conclusions
Results of the BACH study show that nocturia is associated with decreased QOL, increased symptom bother, and an increased prevalence of depression, especially in younger men and women. Similarly, significant trends in decreased QOL and increased odds of depression were observed with increasing number of nightly voids. These results support previous findings suggesting that voiding two or more times nightly is a threshold value of nocturia beyond which the disorder has adverse effects on QOL and well-being.
Acknowledgments
Funding/Support and role of the sponsor: This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) DK 56842. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. NIDDK was not involved in the design and conduct of the study; data collection; data management; analysis; interpretation of results; or preparation, review, and approval of this manuscript. Analyses for the current manuscript were supported through a grant to New England Research Institutes, Inc, from Ferring Pharmaceuticals.
Footnotes
Author contributions: Varant Kupelian had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kupelian, Rosen, McKinlay.
Acquisition of data: McKinlay.
Analysis and interpretation of data: Kupelian, Rosen, Wei, O’Leary, Norgaard, McKinlay .
Drafting of the manuscript: Kupelian.
Critical revision of the manuscript for important intellectual content: Kupelian, Rosen, Wei, O’Leary, Norgaard, McKinlay .
Statistical analysis: Kupelian.
Obtaining funding: McKinlay, Rosen.
Administrative, technical, or material support: Kupelian.
Supervision: Rosen.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Raymond Rosen is a consultant for Pfizer, Eli Lilly, Bayer Shering, Sanofi Aventis, and Boehringer Ingelheim. Jens Peter Norgaard is an employee of Ferring Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–54. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- [2].van Dijk L, Kooij DG, Schellevis FG. Nocturia in the Dutch adult population. BJU Int. 2002;90:644–8. doi: 10.1046/j.1464-410x.2002.03011.x. [DOI] [PubMed] [Google Scholar]
- [3].Tikkinen KA, Tammela TL, Huhtala H, Auvinen A. Is nocturia equally common among men and women? a population based study in Finland. J Urol. 2006;175:596–600. doi: 10.1016/S0022-5347(05)00245-4. [DOI] [PubMed] [Google Scholar]
- [4].Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2002;90:533–6. doi: 10.1046/j.1464-410x.2002.02975.x. [DOI] [PubMed] [Google Scholar]
- [5].Ohayon MM. Nocturnal awakenings and comorbid disorders in the American general population. J Psychiatr Res. 2008;43:48–54. doi: 10.1016/j.jpsychires.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [6].Bing MH, Moller LA, Jennum P, Mortensen S, Skovgaard LT, Lose G. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60–80 years. BJU Int. 2006;98:599–604. doi: 10.1111/j.1464-410X.2006.06390.x. [DOI] [PubMed] [Google Scholar]
- [7].Tikkinen KA, Johnson TM, II, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? a population-based study in Finland. Eur Urol. 2010;57:488–96. doi: 10.1016/j.eururo.2009.03.080. [DOI] [PubMed] [Google Scholar]
- [8].Asplund R. Mortality in the elderly in relation to nocturnal micturition. BJU Int. 1999;84:297–301. doi: 10.1046/j.1464-410x.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- [9].Hakkinen JT, Shiri R, Koskimaki J, Tammela TL, Auvinen A, Hakama M. Depressive symptoms increase the incidence of nocturia: Tampere aging male urologic study (TAMUS) J Urol. 2008;179:1897–901. doi: 10.1016/j.juro.2008.01.037. [DOI] [PubMed] [Google Scholar]
- [10].Johnson TV, Abbasi A, Ehrlich SS, Kleris RS, Raison CL, Master VA. Nocturia associated with depressive symptoms. Urology. 2011;77:183–6. doi: 10.1016/j.urology.2010.04.048. [DOI] [PubMed] [Google Scholar]
- [11].Robertson C, Link CL, Onel E, et al. The impact of lower urinary tract symptoms and comorbidities on quality of life: the BACH and UREPIK studies. BJU Int. 2007;99:347–54. doi: 10.1111/j.1464-410X.2007.06609.x. [DOI] [PubMed] [Google Scholar]
- [12].McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389–96. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fitzgerald MP, Link CL, Litman HJ, Travison TG, McKinlay JB. Beyond the lower urinary tract: the association of urologic and sexual symptoms with common illnesses. Eur Urol. 2007;52:407–15. doi: 10.1016/j.eururo.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fitzgerald MP, Litman HJ, Link CL, McKinlay JB. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH Survey. J Urol. 2007;177:1385–9. doi: 10.1016/j.juro.2006.11.057. [DOI] [PubMed] [Google Scholar]
- [15].Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- [16].Epstein RS, Deverka PA, Chute CG, et al. Validation of a new quality of life questionnaire for benign prostatic hyperplasia. J Clin Epidemiol. 1992;45:1431–45. doi: 10.1016/0895-4356(92)90205-2. [DOI] [PubMed] [Google Scholar]
- [17].Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- [18].Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- [19].Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–27. [PMC free article] [PubMed] [Google Scholar]
- [20].Cochran W. Sampling techniques. ed 3 John Wiley and Sons; New York, NY: 1977. [Google Scholar]
- [21].Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15:141–55. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- [22].Dugan E, Roberts CP, Cohen SJ, et al. Why older community-dwelling adults do not discuss urinary incontinence with their primary care physicians. J Am Geriatr Soc. 2001;49:462–5. doi: 10.1046/j.1532-5415.2001.49094.x. [DOI] [PubMed] [Google Scholar]
- [23].Wolters R, Wensing M, van Weel C, van der Wilt GJ, Grol RP. Lower urinary tract symptoms: social influence is more important than symptoms in seeking medical care. BJU Int. 2002;90:655–61. doi: 10.1046/j.1464-410x.2002.02996.x. [DOI] [PubMed] [Google Scholar]
- [24].Zhang X, Zhang J, Chen J, et al. Prevalence and risk factors of nocturia and nocturia-related quality of life in the Chinese population. Urol Int. 2011;86:173–8. doi: 10.1159/000321895. [DOI] [PubMed] [Google Scholar]
- [25].Helfand BT, McVary KT, Meleth S, et al. The relationship between lower urinary tract symptom severity and sleep disturbance in the CAMUS trial. J Urol. 2011;185:2223–8. doi: 10.1016/j.juro.2011.02.012. [DOI] [PubMed] [Google Scholar]
- [26].Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- [27].Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the Osteoporotic Fractures in Men sleep study. J Am Geriatr Soc. 2008;56:1665–73. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768–74. doi: 10.1001/archinte.166.16.1768. [DOI] [PubMed] [Google Scholar]
- [29].Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- [30].Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- [31].Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- [32].Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147:969–77. doi: 10.1093/oxfordjournals.aje.a009387. [DOI] [PubMed] [Google Scholar]
- [33].St Sauver JL, Hagen PT, Cha SS, et al. Agreement between patient reports of cardiovascular disease and patient medical records. Mayo Clin Proc. 2005;80:203–10. doi: 10.4065/80.2.203. [DOI] [PubMed] [Google Scholar]