Abstract
BACKGROUND
Protein is absorbed primarily as di/tripeptides which are transported into the enterocyte exclusively by H+/peptide cotransporter 1 (PEPT1). Diurnal changes in expression and function of several other mucosal transporters occur in rat.
HYPOTHESIS
Diurnal variations in mRNA, protein, and transport function of PEPT1 occur in rat duodenum and jejunum but not in ileum.
METHODS
Mucosal levels of mRNA and protein were determined at 9AM, 3PM, 9PM, and 3AM (n=6 each) by real time RT-PCR and Western blotting, respectively, in rats maintained in a 12-h light/dark room [lights 6AM to 6PM]; transporter-mediated uptake of di-peptide (Gly-Sar) was also measured by everted sleeve technique.
RESULTS
mRNA transcripts of PEPT1 and Gly-Sar uptake varied diurnally in duodenum and jejunum (peak at 3PM, p<0.05) but not in ileum; maximal uptake was in jejunum. Vmax (nmol/cm/min) was greater at 3PM and 9PM compared to 9AM (3PM vs. 9AM: 104 vs. 62 in duodenum, and 185 vs. 101 in jejunum; p<0.03); Km was unchanged across time points or locations. Protein levels varied minimally in jejunum and ileum with peaks at 9PM and 3AM.
CONCLUSION
Gene expression and transport function of PEPT1 vary diurnally in duodenum and jejunum in temporal association with nocturnal feeding of rats.
Keywords: peptide transporter 1, PEPT1, diurnal rhythm, rat, small intestine, protein absorption, short peptides
INTRODUCTION
Dietary proteins are digested in the lumen of the small intestine into a mixture of primarily short peptides and some free amino acids. Absorption of protein digestion products occurs predominantly as di- and tri-peptides rather than individual amino acids (1 - 3). Proton-dependent peptide transporter 1 (PEPT1) is the exclusive peptide transporter expressed in the brush border (apical membrane) of enterocytes and mediates the uptake of essentially all di- and tri-peptides from the lumen (3 - 5). PEPT1 plays important roles not only as a nutrient transporter but also as a drug transporter for several peptide-like drugs (e.g. β-Lactam antibiotics) (5 - 6).
Various factors regulate gene expression and transport function of mucosal transporters, such as luminal substrates, hormones, and ontogeny (7 - 9). A diurnal rhythm in gene expression and transport function of several other mucosal transporters (e.g. hexose transporters) occurs in the proximal intestine of rodents (i.e. duodenum and jejunum) in coordination with their nocturnal feeding pattern (10 - 13). Our aim was to look for and characterize diurnal variations in expression and function of PEPT1 throughout the rat small intestine. Identifying temporal and segmental variations in expression and transport function of nutrient transporters may allow us to modulate their regulatory mechanisms during various diseases or after surgical intervention. Our hypothesis was that diurnal variations in gene expression (mRNA and protein) and transport function of PEPT1 occur in rat duodenum and jejunum but not in ileum.
METHODS
After approval from our Institutional Animal Care and Use Committee and in accordance with the NIH guidelines for the humane use and care of laboratory animals, 24 male Lewis rats weighing 250-300 g (Harlan, Indianapolis, IN) maintained in a controlled, 12-h light/dark room (lights on from 6AM to 6PM) with free access to water and standard rat chow (5001 Rodent Diet, PMI Nutrition International LLC, Brentwood, MO) were acclimated for at least 1 week. Feeding patterns were determined by measuring food consumption twice daily (at 6AM and 6PM) in 12 rats for 1 wk prior to sacrifice. To determine diurnal rhythmicity in expression and function of PEPT1, 6 rats at each of four time points (9AM, 3PM, 9PM, 3AM) were killed, and the levels of mRNA, protein, and transport activity for PEPT1 were measured in duodenum, jejunum, and ileum.
Tissue Harvest
Rats were anesthetized using inhaled 2% isoflurane and intraperitoneal pentobarbital (50 mg/kg). After celiotomy, the duodenum was cannulated just distal to pylorus, and the small intestine was flushed with cold (4°C) Ringer’s solution. The small intestine was excised and placed immediately in cold (4°C), oxygenated (95% O2/ 5% CO2) Ringer’s solution. The proximal duodenum was used for everted sleeves (see below, Uptake Function), and the distal duodenum was used for mRNA and protein measurements. Similarly, mid-jejunum and midileum were studied. The mucosa was scraped bluntly using a glass slide into cold, phosphate-buffered saline (PBS). Samples for mRNA analysis were placed in RNA stabilization buffer (RNALater, Qiagen, Valencia, CA) and stored immediately at -80°C. The samples for protein analysis were collected separately, placed in cold RIPA buffer containing protease inhibitors (Pierce, Rockford, IL), and stored at -80°C. For histomorphometry, 0.5 cm portions of each anatomic segment were pinned on a support and fixed in 10% buffered formalin.
mRNA Measurement
We used real-time, reverse transcription, polymerase chain reaction (RT-PCR) to quantitate mRNA levels for PEPT1. Mucosal samples stored in RNA stabilization buffer were thawed on ice and homogenized; RNA was isolated using the RNeasy Midi kit (Qiagen, Valencia, CA). RNA was then reverse transcribed into cDNA using the Super Script III kit (Invitrogen, Carlsbad, CA); cDNA levels of PEPT1 and the stably expressed housekeeping gene glyceraldehyde-6-phosphate dehydrogenase (GAPDH) were then determined using RT-PCR in a 7500 Thermocycler using Taqman® chemistries with primers and fluorescently-labeled probes in assay mixes (Applied Biosystems, San Francisco, CA). Standard curves from serial dilutions of known copy numbers were used to calculate copy numbers of cDNA for each sample. All samples were run as duplicates with 2 μl of cDNA added to 23 μl of master mix. RT-PCR was carried out at 95°C for 10 min followed by 40 cycles of 15 s at 95° and 1 min at 60°C after which fluorescence measurements were made. Transporter copy numbers were normalized to copy numbers of GAPDH from each sample.
Protein Measurement
Western blotting was used to measure semi-quantitatively the levels of total cellular protein for PEPT1. Tissue samples stored in RIPA buffer containing protease inhibitors were thawed on ice and placed in RIPA lysis buffer containing protease inhibitors in attempt to minimize protein degradation. Samples were homogenized using a Kontes Pellet Pestle (Fischer Scientific, Pittsburg, PA). The protein-containing supernatant was separated by centrifugation at 5000 × g for 15 min. Protein concentrations were measured by the bicinchoninic acid method (Pierce, Rockford, IL); 200 μg of protein was resolved on a 10% SDS-PAGE gel (Bio-Rad, Hercules, CA) and transferred electrically to a PVDF membrane (Millipore, Bedford, MA). Membranes were blocked using 5% milk in tris-buffered saline with Tween (TBS-T). GAPDH was used as a stably expressed “housekeeping” protein. Membranes were incubated overnight at 4°C with primary antibody for PEPT1 (Santa Cruz Biotechnology, Santa Cruz, CA), and GAPDH antibody (US Biological, Swampscott, MA). After incubation with primary antibody, membranes were rinsed 3 times with TBS-T and incubated with secondary antibody in TBS-T containing 5% milk using horseradish peroxidase-conjugated, goat anti-rabbit IgG for PEPT1 and anti-mouse IgG for GAPDH (Sigma, St. Louis, MO). Protein bands visualized with a colorimetric reaction using Opti-4CN Substrate kits (Bio-Rad) were scanned, and Scion Image (Scion Corp, MA) was used for semi-quantitative measurements based on band densitometry. Protein measurements were normalized to GAPDH to estimate the amount of protein per enterocyte.
Uptake Function
We measured transporter-mediated uptake of the di-peptide Glycyl-Sarcosine (Gly-Sar), a non hydrolysable substrate for PEPT1 (14), using a modified everted sleeve technique described by Karasov and Diamond (15). Intestinal segments (1 cm) were everted over a pre-grooved steel rod and secured with silk ties, thereby exposing the mucosal surface externally. Sleeves were kept in chilled (4°C) Ringer’s solution bubbled with 95% O2/5% CO2. The sleeves were transferred into 8 ml of warmed (38°C) Gly-Sar-free incubation medium (in mM: 129 NaCl, 5.1 KCL, 1.4 CaCl2, 1.3 NaH2PO4, and 1.3 Na2HPO4; pH 6.0) (14) for 5 min bubbled with 95% O2/5% CO2, and then placed in 8 ml of 38°C incubation medium with iso-osmotic replacement of NaCl using 0.02, 1, 5, 20, or 40 mM Gly-Sar and stirred at 1,200 rpm. One μCi of 14C-Gly-Sar was included in the test solution to measure total uptake of Gly-Sar, from which the transporter-mediated uptake by PEPT1 was calculated (see below). After 1-min incubation, tissues were removed, rinsed in 30 ml of ice-cold (Gly-Sar-free) incubation medium stirred at 1,200 rpm for 20 s, placed in glass scintillation vials containing 1 ml of tissue solubilizer (Perkin-Elmer, Boston, MA), and kept in a 50°C water bath for 3 h. After complete solubilization, 15 ml of scintillation counting cocktail (Opti-Flour, Perkin-Elmer, CT) was added, and DPMs of 14C were determined using liquid scintillation techniques.
Transporter vs. Non-Transporter Mediated Uptake
To calculate transporter-mediated uptake of Gly-Sar, total uptake needed to be corrected for passive diffusion and adherent Gly-Sar to the mucosal surface (non-transporter mediated “uptake”). Based on methods of Matthews et al (16), non-transporter mediated uptake at lesser concentrations can be best estimated from observed uptake at greater concentrations. As the substrate concentration increases, non-transporter mediated passive uptake increases linearly before and after the transporter is saturated; thus the linear increase in total uptake after the transporter is saturated is attributed “only” to non-transporter mediated “uptake”, i.e. passive diffusion and mucosal adherence. We used 20 and 40 mM concentrations of Gly-Sar (at which a linear increase in total uptake was observed) to estimate non-transporter mediated “uptake” at lesser concentrations (0.02, 1, 5 mM). Subtraction of estimated, non-transporter mediated uptake from observed total uptake allowed estimation of the transporter-mediated uptake.
Villous Height
Formalin-fixed tissues were embedded in paraffin, sectioned parallel to the villous axis, and stained with hematoxylin and eosin. Maximum villous height was measured from above the crypt to the tip of the villous at 10 times magnification using an optical reticule with a micrometer. Measurements from each specimen were made on at least 6 slides with at least 3 measurements per slide.
Statistical Analysis
All levels of mRNA and protein expression were expressed as the ratio of PEPT1 to the housekeeping gene (GAPDH). Transporter-mediated uptake of Gly-Sar was measured in nmol/cm/min with Lineweaver-Burke plots used to calculate Vmax and Km. Data are reported as median ± interquartile range (IQR). Kruskal-Wallis analysis was used to compare non-parametric data across multiple groups; Wilcoxon rank sums were used for direct comparisons between individual groups. P-values were corrected according to the Bonferroni method, only corrected p values of < 0.05 were considered significant, and n values are number of rats.
RESULTS
Feeding Pattern
Rats followed a nocturnal-based feeding pattern. Greater than 70% of chow intake occurred between 6PM and 6AM (median intake during dark vs. light: 18 vs. 6g; p<0.0001).
Expression of mRNA and Protein
mRNA and protein levels of PEPT1 showed site-specific, diurnal variations; mRNA expression varied diurnally in duodenum and jejunum, whereas total cellular protein for PEPT1 exhibited diurnal variations in jejunum and ileum. A delay of 12 h in time was shown between peaks in mRNA and protein levels.
Duodenum
mRNA levels of PEPT1 followed a diurnal rhythm in duodenum with peak levels at 3PM (p<0.002; Figure1A). The median relative fold change (peak over minimum level) was about 5-fold. Protein levels of PEPT1 did not vary diurnally (p=0.48; Figure 1A).
FIGURE 1.
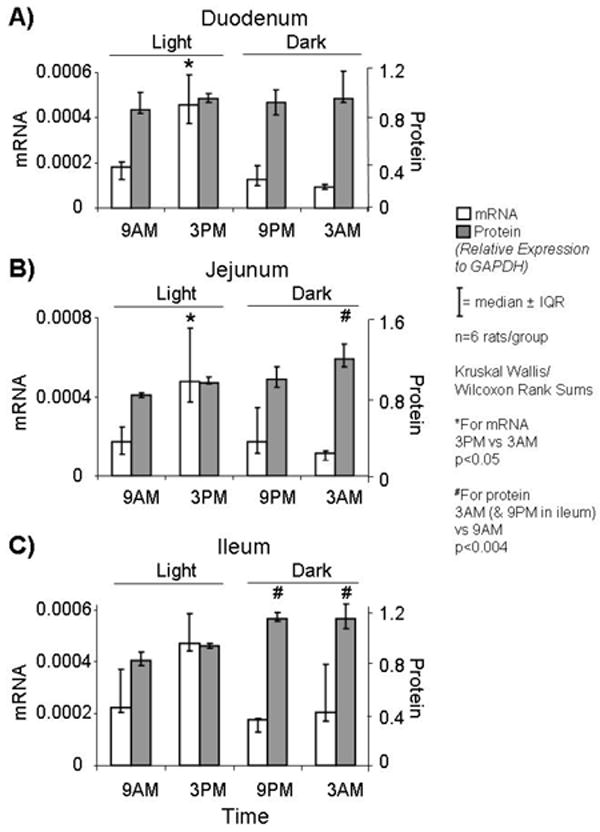
Variations in mRNA and protein expression levels of PEPT1 in A) duodenum, B) jejunum, and C) ileum at 4 time-points during the day. mRNA expression varied diurnally in duodenum and jejunum (~5 folds). Protein levels showed less impressive diurnal variations in jejunum and ileum (<1.5 folds).
Jejunum
Similar to the pattern exhibited in duodenum, mRNA levels of PEPT1 varied diurnally in jejunum with peak levels at 3PM (p<0.05; Figure 1B). The median relative fold-change was about 4-fold. Also, protein expression showed diurnal variations with peak levels at 3AM (p<0.004; Figure 1B), a 12-h delay from peak in mRNA. The relative fold change in protein was much less impressive (about a 1.5-fold increase at 3AM).
Ileum
Expression of mRNA did not show significant diurnal variation across time points in ileum (p=0.2; Figure 1C); protein levels were increased at 9PM and 3AM compared to 9AM (p<0.004; Figure 1C), but, although statistically significant, the fold change was small (1.4-fold).
There was no difference between the anatomic segments at any time point in the relative expression of mRNA and protein per enterocyte (p>0.2; Figure 2A-B).
FIGURE 2.
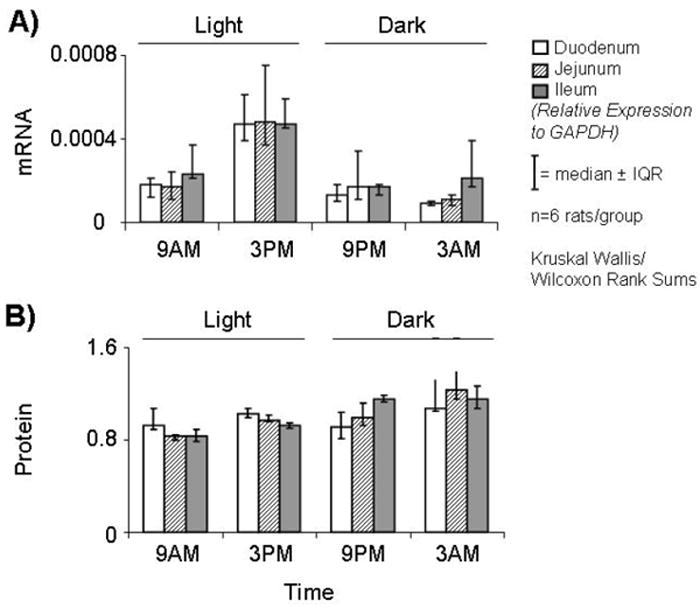
Relative expression levels of A) mRNA and B) protein of PEPT1 in the three anatomic segments at 4 time-points. At each time point, there was no difference between the intestinal segments in relative expression of mRNA and protein per enterocyte.
Transporter-Mediated uptake of Gly-Sar
Uptake in all three segments demonstrated saturation kinetics consistent with transporter-mediated uptake. In duodenum and jejunum, uptake of Gly-Sar varied diurnally with values greater at 3PM and 9PM compared to 9AM for all concentrations (p<0.05; Figure 3A-B). In ileum there was no diurnal variation in uptake of Gly-Sar across time points (p>0.5; Figure 3C). Similar to uptake values, calculated Vmax (in nmol/cm/min) varied diurnally in duodenum and jejunum but not in ileum (3PM vs. 9AM: 104 vs. 62 in duodenum, and 185 vs. 101 in jejunum, p<0.03; Figure 4A); Km did not differ amongst the segments at all time points (p>0.2; Figure 4B). Values for uptake and calculated Vmax in jejunum were greater (>1.5 fold) than in duodenum and ileum at all time points; uptake in duodenum and ileum, however, were quite similar at 3AM and 9AM, but greater uptake was shown in duodenum at 3PM and 9PM.
FIGURE 3.
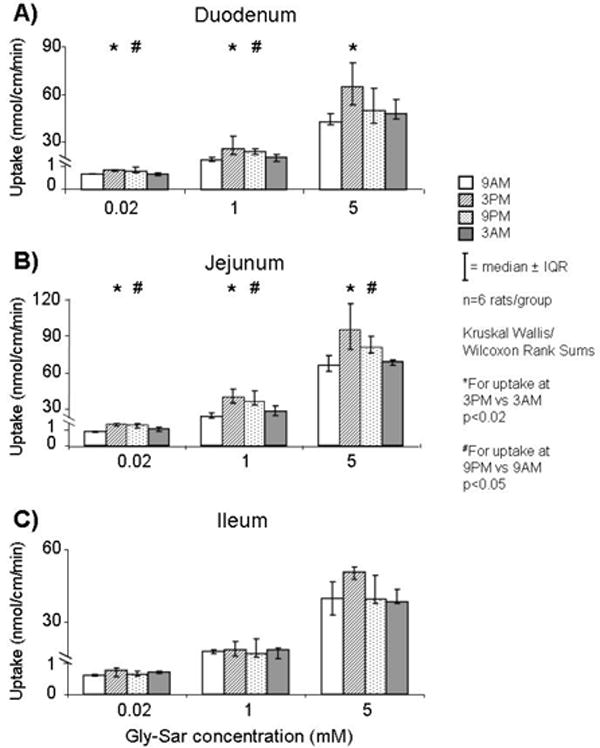
Transporter-mediated uptake of Gly-Sar (at three concentrations) in A) duodenum, B) jejunum, and C) ileum at 4 time-points. Gly-Sar uptake varied diurnally in duodenum and jejunum but not in ileum.
FIGURE 4.
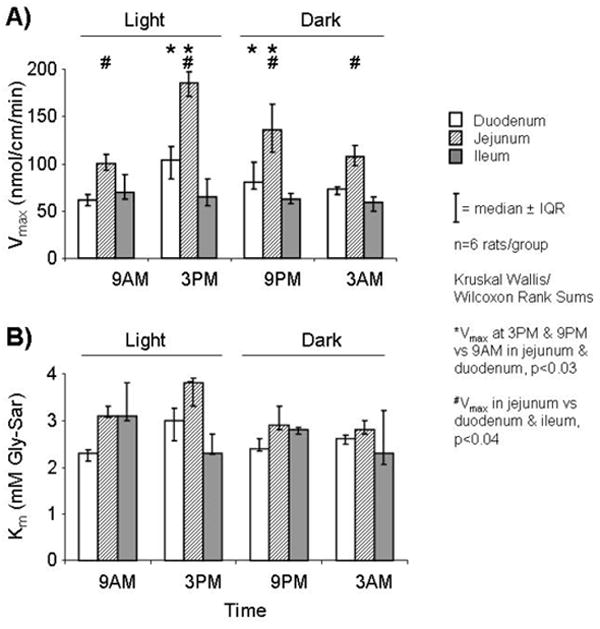
Values for A) Vmax and B) Km in each intestinal segment at 4 time-points. Vmax varied diurnally in duodenum and jejunum but not in ileum; Km remained unchanged.
Villous Height
Median villous height was greater in duodenum and jejunum compared to ileum (0.47 and 0.48 vs. 0.29 mm, respectively; p<0.0001).
DISCUSSION
The physiologic and clinical importance of intestinal PEPT1 has been well recognized only recently. Several groups have studied the mechanisms regulating expression and function of PEPT1; however, few studies investigated diurnal rhythm and only in rat duodenum (14). To the best of our knowledge, there have been no comprehensive reports of a diurnal expression and function of PEPT1 at a segmental level in rat small intestine nor a characterization of its transport capacity (Vmax) and affinity (Km) in each anatomic segment.
We demonstrated a nocturnal-based feeding pattern of rats (>70 % of food intake occurred during dark cycle); this pattern of feeding correlated with the gene expression of PEPT1 as well as with its transport function. In duodenum and jejunum, the levels of mRNA peaked in anticipatory fashion 3h before the dark cycle during which most feeding occurred; this increased transcription coincided with and was followed by an increase in the transport function of PEPT1 which peaked just before the onset of dark cycle and remained increased through the initial dark phase. The simultaneity between peaks of mRNA and transport function of PEPT1 suggests the presence of an anticipatory mechanism inducing transcription of new transporter mRNA, and paralleled possibly with recruitment of preformed transporters from cytoplasmic stores to the apical membrane to further increase uptake. This latter concept of translocation of pre-synthesized, cytosolic transporter to the apical membrane would be most consistent with our findings of minimal diurnal changes in total cellular protein for PEPT1 and no simultaneous peak in total cellular protein at the times when mRNA levels are greatest. Others have shown similar temporal associations between gene expression and function of PEPT1 in rat duodenum (14).
Indeed, increased uptake was associated temporally with an increase in Vmax, a function of the number of apical transporters participating actively in uptake, which was shown in both duodenum (1.7-fold) and jejunum (1.8-fold); in addition, the Km (a function of the receptor affinity for its substrate) remained unchanged at all time points, reinforcing the concept that increased uptake is not a result of a change in type of transporter or changes in protein conformation, but rather an increase in the number of transporters expressed in the apical membrane. In ileum, however, there was no significant diurnal variation in mRNA levels or transport function of PEPT1; these findings are similar to the pattern exhibited by hexose transporters in ileum (10).
Levels of total cellular protein for PEPT1, at least as measured by our technique of isolation and semi-quantitative Western blot, showed a slight albeit statistically significant increase during the dark phase in jejunum and ileum (<1.5-fold changes). In jejunum, peak levels of total protein displayed a 12-h delay from peak in mRNA; this delay may reflect the time needed for translation. The peak in total cellular protein, however, was dissociated temporally from the peak in measured uptake (and presumably the peak in number of apical transporters), possibly representing posttranscriptional and/or posttranslational processing of PEPT1, such as activation of transporters (phosphorylation, glycosylation, etc.) or more likely the translocation of preformed transporters “from pre-synthesized cytosolic pools” to the apical, brush border, membrane. Measuring protein levels of PEPT1 expressed in the brush border (in addition to measuring total cellular PEPT1) would give better elucidation for these possibilities; however, lack of a stably expressed housekeeping gene in the apical membrane (for normalization of expression), and the potential alterations incurred by preparing brush border (apical) membrane vesicles (BBMVs), question the reliability of these methods.
An interesting finding was the lack of difference between the three anatomic segments in relative expressions of mRNA and total protein for PEPT1 per enterocyte. Furthermore, there was no difference in the baseline uptake of Gly-Sar (per 1-cm segments) between duodenum and ileum at 3AM and 9AM, whereas uptake in jejunum was greater than in duodenum and ileum. We expected to find a decrease in expression and function of PEPT1 aborally (especially in ileum) similar to the pattern exhibited by hexose transporters (10); however, our findings might suggest an important role for PEPT1 in the distal small bowel, possibly to recover undigested protein and protein liberated from mucosal cells being sloughed along the gut tract.
Villous height (a rough indicator for number of enterocytes per segment) in the three anatomic segments did not correlate with functional activity in Gly-Sar uptake; there were no differences between jejunum and duodenum in villous height nor in total gene expression (per cell), but the functional activity (uptake) was greater in jejunum. This observation might suggest the presence of other factors affecting the relative absorption of short peptides in each segment, possibly via a variant distribution of apical transporters in the brush border of enterocytes (relative to total cellular transporters) in each segment or a differential distribution of the population of transporters along the crypt-villous axis; differences in distribution of peptide transporters along the villi were reported previously in rat jejunum through measurements of immunohistochemistry showing a relative increase in expression toward the tip of villous (17). Furthermore, immunofluorescence localization of PEPT1 in the brush border, as conducted by other groups, showed that the rat jejunum (compared to duodenum and ileum) gives the strongest staining and, presumably, has greater numbers of transporters at the apical membrane to participate in uptake (18), which is most consistent with our finding of maximal uptake activity in the jejunum segment.
In summary, our study demonstrates that gene expression and transport function of PEPT1 follow a diurnal pattern, especially in duodenum and jejunum, in temporal association with the nocturnal feeding of rats; uptake activity for PEPT1 also varied at a segmental level. Several clinical implications can be gleaned by identifying the temporal and segmental variations in expression and function of PEPT1 in the small intestine, including studies of drug therapy, enteral nutrition, and management of post-surgical malabsorption.
Acknowledgments
We would like to thank the Mary Elizabeth Groff Surgical Medical Research and Education Charitable Trust for the generous funding in support of this work. Also, we thank Dr. Ken-ichi Inui and his research group (Kyoto University, Kyoto, Japan) for providing our lab with the cDNA of rat PEPT1, and Deborah Frank for her secretarial expertise.
Research supported by a grant from the Mary E. Groff Foundation and R01 DK 39337-18 from the National Institutes of Health (MGS)
Footnotes
Presented in part at the Academic Surgical Congress 4th Annual Meeting, Fort Myers, FL on February 6, 2009, and published in abstract form in Journal of Surgical Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matthews DM, Adibi SA. Peptide absorption. Gastroenterology. 1976;71:151–161. [PubMed] [Google Scholar]
- 2.Leibach FH, Ganapathy V. Peptide transporters in the intestine and the kidney. Ann Rev Nutr. 1996;16:99–119. doi: 10.1146/annurev.nu.16.070196.000531. [DOI] [PubMed] [Google Scholar]
- 3.Adibi SA. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113:332–340. doi: 10.1016/s0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- 4.Fei YJ, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- 5.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Ann Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 6.Saito H, Okuda M, Terada T, Sasaki S, Inui K. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of β-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther. 1995;275:1631–1637. [PubMed] [Google Scholar]
- 7.Erickson RH, Gum JR, Lindstrom MM, Mckean D, Kim YS. Regional expression and dietary regulation of rat small intestinal peptide and amino acid transporter mRNAs. Biochem Biophys Res Commun. 1995;216:249–257. doi: 10.1006/bbrc.1995.2617. [DOI] [PubMed] [Google Scholar]
- 8.Thamotharan M, Bawani SZ, Zhou XD, Adibi SA. Hormonal regulation of oligopeptide transporter PEPT1 in a human intestinal cell line. Am J Physiol. 1999;276:C821–C826. doi: 10.1152/ajpcell.1999.276.4.C821. [DOI] [PubMed] [Google Scholar]
- 9.Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol. 2003;285:G779–G788. doi: 10.1152/ajpgi.00056.2003. [DOI] [PubMed] [Google Scholar]
- 10.Houghton SG, Duenes JA, Fatima J, Iqbal CW, Kasparek MS, Sarr MG. Coordinated, diurnal hexose transporter expression in rat small bowel: implications for small bowel resection. Surgery. 2008;143:79–93. doi: 10.1016/j.surg.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balakrishnan A, Stearns AT, Rounds J, Irani J, Giuffrida M, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1) Surgery. 2008;143:813–818. doi: 10.1016/j.surg.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan X, Terada T, Okuda M, Inui K. The diurnal rhythm of the intestinal transporters SGLT1 and PEPT1 is regulated by the feeding conditions in rats. J Nutr. 2004;134:2211–2215. doi: 10.1093/jn/134.9.2211. [DOI] [PubMed] [Google Scholar]
- 13.Fatima J, Iqbal CW, Houghton SG, Kasparek MS, Duenes JA, Zheng Y, Sarr MG. Hexose transporter expression and function in mouse small intestine: role of diurnal rhythm. J Gastrointest Surg. doi: 10.1007/s11605-008-0776-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan X, Terada T, Irie M, Saito H, Inui K. Diurnal rhythm of H+-peptide cotransporter in rat small intestine. Am J Physiol. 2002;283:G57–G64. doi: 10.1152/ajpgi.00545.2001. [DOI] [PubMed] [Google Scholar]
- 15.Karasov WH, Diamond JM. A simple method for measuring intestinal solute uptake in vitro. J Comp Physiol. 1983;152:105–116. [Google Scholar]
- 16.Matthews DM, Grandy RH, Taylor E, Burston D. Influx of two dipeptides, glycylsarcosine and L-glutamyl-L-glutamic acid, into hamster jejunum in vitro. Clin Sci. 1979;56:15. doi: 10.1042/cs0560015. [DOI] [PubMed] [Google Scholar]
- 17.Ogihara H, Suzuki T, Inui K, Takata K. Peptide transporter in the rat small intestine: ultrastructural localization and the effect of starvation and administration of amino acids. Histochem J. 1999;31:169–174. doi: 10.1023/a:1003515413550. [DOI] [PubMed] [Google Scholar]
- 18.Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun. 1996;220(3):848–52. doi: 10.1006/bbrc.1996.0493. [DOI] [PubMed] [Google Scholar]


