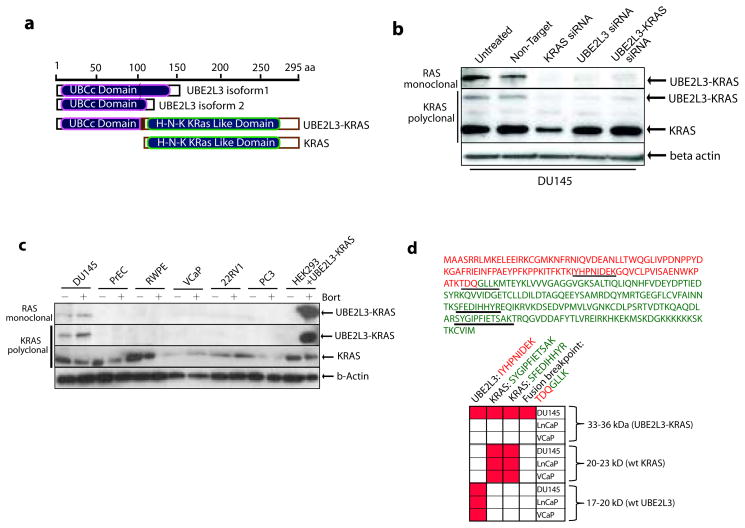
Figure 2. Characterization of the UBE2L3-KRAS fusion protein.
(a) Schematic representations of UBE2L3, KRAS, and the predicted UBE2L3-KRAS fusion protein. (b) Expression of the UBE2L3-KRAS fusion protein in DU145 cells. Immunoblot analysis of DU145 cells using an anti-RAS mouse monoclonal antibody and an anti-KRAS rabbit polyclonal antibody detects a 33kDa fusion protein specific to DU145 cells. siRNA duplexes employed are indicated. β-actin was used to demonstrate equal loading. (c) Survey of the UBE2L3-KRAS fusion protein in a panel of prostate cancer cell lines and stabilization of protein expression with a proteosome inhibitor, bortezomib. Cell lines are indicated and treated in the presence or absence of 500nM bortezomib for 24 hours. HEK293 cells were transfected with an expression construct encoding UBE2L3-KRAS. Immunoblot analysis was carried out using KRAS polyclonal and RAS monoclonal antibodies. (d) Mass spectrometric assay for the detection of the UBE2L3-KRAS protein in DU145 cells. An MRM-MS assay was developed to detect the UBE2L3-KRAS fusion protein. Upper panel, sequence of the UBE2L3-KRAS fusion protein with amino acids colored in red from UBE2L3 and colored in green from KRAS. Tryptic peptides used for MRM-MS analysis are underlined. Matrix represents positive measurement (highlighted in red) of peptides from corresponding gel fractions of DU145, LNCaP, and VCaP whole cell lysates.