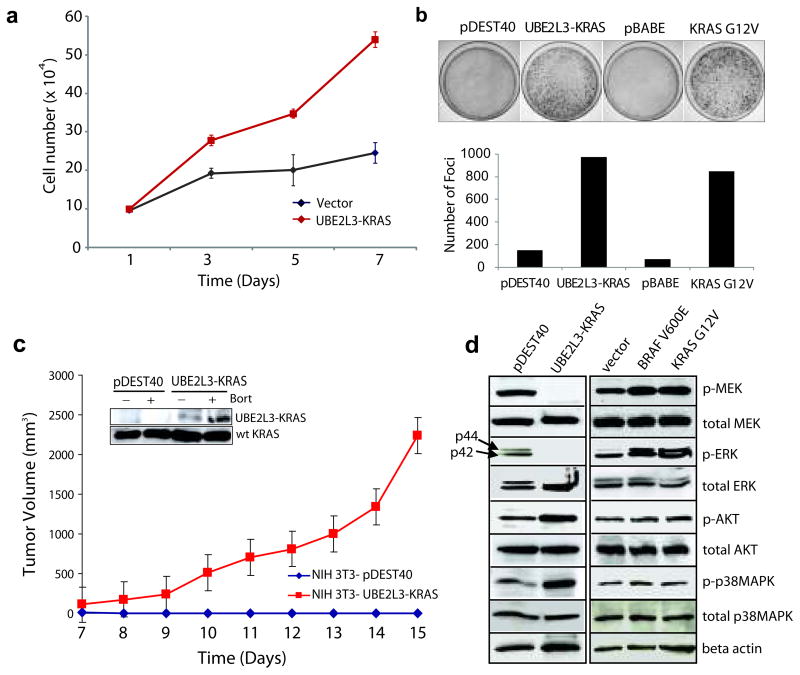
Figure 3. Transforming activities of the UBE2L3-KRAS fusion in NIH 3T3 cells.
(a) Overexpression of UBE2L3-KRAS in NIH 3T3 cells increases cellular proliferation. pDEST40 represents an empty vector. (b) Overexpression of UBE2L3-KRAS in NIH 3T3 cells induces focus formation. Oncogenic KRAS G12V was used as a positive control with respective empty vectors as negative controls (pDEST40 and pBABE). Photographs of representative plates are shown in the upper panel and quantification of focus formation is shown by the bar graph in the lower panel. (c) UBE2L3-KRAS transfected NIH 3T3 cells form tumors in nude mice. Stable polyclonal populations of NIH 3T3 cells expressing either the vector or UBE2L3-KRAS fusion gene were injected subcutaneously into nude mice. Tumor growth was monitored from day 7 to day 15 as indicated. The insert shows the presence of the fusion protein in the stably transfected NIH 3T3 cells, which is further stabilized upon bortezomib treatment. (d) Investigation of the downstream signaling pathways engaged by the UBE2L3-KRAS fusion in NIH 3T3 cells. Lysates prepared from stably transfected NIH 3T3 polyclonal populations and vector controls were subject to immunoblot analysis for phospho- and total MEK, ERK, AKT, and p38 MAPK. Oncogenic BRAFV600E and KRASG12V were included as controls. β-actin was used as a loading control.