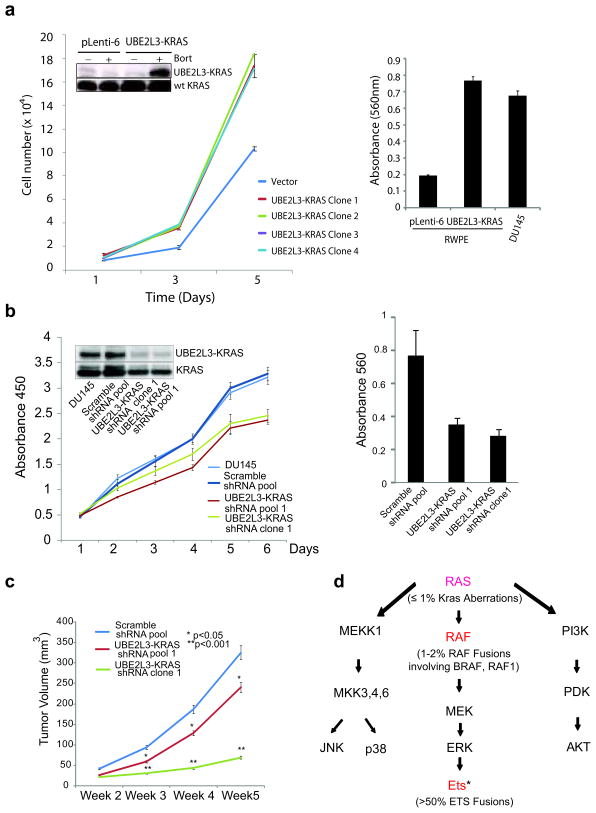
Figure 4. The oncogenicity of UBE2L3-KRAS fusion in the prostate context.
(a) Expression of the UBE2L3-KRAS fusion in RWPE benign prostate epithelial cells leads to increased cellular proliferation and invasion. Left, the results of cell proliferation assays using stable RWPE cell clones infected with either the pLenti-6 vector or UBE2L3-KRAS. The inset shows the 33kd fusion protein detected only in the fusion transfected cells treated with bortezomib to enhance protein stability (data from a representative clone is shown (Clone 2)). Right, modified Boyden chamber-matrigel assays using the pLenti-6 vector and the fusion expressing cells (Clone 2). Invading cells were stained with crystal violet and quantitated. DU145 prostate cancer cells were used as a positive control. (b) Knockdown of the UBE2L3-KRAS fusion reduces cell proliferation and invasion in DU145 cells. Left, cell growth relative to the control shRNA was monitored using WST-1 assay for 6 days. Insert shows the immunoblot analysis for the 33kd fusion protein detected using Ras monoclonal antibody. Right, results of matrigel invasion assay for DU145 pool and clone with UBE2L3-KRAS knock-down. Scrambled shRNA duplexes are used as control. (c) Knock-down of the UBE2L3-KRAS fusion attenuates prostate tumor growth in mouse xenograft models. The figure shows a plot of mean tumor volume trajectories over time for mice inoculated with DU145 pool (red) or single clone (green) after UBE2L3-KRAS stable knock-down. Error bars represent the standard error of the mean at each time point. (d) A summary of RAS-RAF signaling pathways in relation to recurrent gene fusions characterized in prostate cancer. Genes that participate in fusion events are indicated in red. In parenthesis are the percentage of prostate cancers harboring aberrations in the ETS family, RAF family, and KRAS gene locus. * ETS family members involved in gene fusions include ERG, ETV1, 4, and 5. Figure adapted and modified from: Gioeli, Kraus, Weber et al, Current Clinical Oncology: Prostate Cancer.