Abstract
Genetic modification is continuing to be an essential tool in studying stem cell biology and in setting forth potential clinical applications of human embryonic stem cells (HESCs)1. While improvements in several gene delivery methods have been described2-9, transfection remains a capricious process for HESCs, and has not yet been reported in human induced pluripotent stem cells (iPSCs). In this video, we demonstrate how our lab routinely transfects and nucleofects human iPSCs using plasmid with an enhanced green fluorescence protein (eGFP) reporter. Human iPSCs are adapted and maintained as feeder-free cultures to eliminate the possibility of feeder cell transfection and to allow efficient selection of stable transgenic iPSC clones following transfection. For nucleofection, human iPSCs are pre-treated with ROCK inhibitor11, trypsinized into small clumps of cells, nucleofected and replated on feeders in feeder cell-conditioned medium to enhance cell recovery. Transgene-expressing human iPSCs can be obtained after 6 hours. Antibiotic selection is applied after 24 hours and stable transgenic lines appear within 1 week. Our protocol is robust and reproducible for human iPSC lines without altering pluripotency of these cells.
Keywords: Medicine, Issue 56, Developmental Biology, Transfection, iPS cells, IPSCs, ES cells, HESCs, Nucleofection
Protocol
Our protocol begins with a method to adapt human iPSCs to feeder-free cultures, followed by protocols for transfecting human iPSCs using GeneJuice (EMD) and nucleofection of human iPSCs using an AMAXA nuclefector device.
Note: The following procedures are performed in a sterile laminar flow hood. All media and solutions are equilibrated to 37°C or room temperature before starting unless otherwise specified.
1. Establishing human iPSCs on feeder-free system
Human iPSCs previously maintained on feeder cells can be split, transferred onto Geltrex-coated dish and maintained for two passages prior to feeder-free transfection.
Thaw Geltrex overnight at 4°C. To prepare Geltrex coating, dilute defrosted Geltrex 1:50 in cold DMEM. Mix the solutions gently.
Note: Geltrex, like Matrigel is a soluble form of basement membrane matrix purified from murine Engelbreth-Holm-Swarm (EHS) tumor cells. Alternatively, Matrigel can be used as an extracellular matrix to establish feeder-free human iPSC cultures.
Cover the whole surface of culture wells with Geltrex solution (1 ml for a 35-mm well). Coat wells with Geltrex at 37°C incubator for 1 hour.
To passage human iPSCs, add 1 ml of accutase per well and incubate at 37°C for 1 min until most cells start to detach.
To passage human iPSCs, add 1 ml of accutase per well and incubate at 37°C for 1 min until most cells start to detach.
Add 10-15 glass beads to the cells and gently swirl the plate. Add 2 ml of KnockOut DMEM/ F12 and gently triturate. Transfer cell suspension to a 10 ml centrifuge tube.
Spin cells at 800 rpm for 3 min at room temperature.
Aspirate supernatant from the tube, leaving human iPSC pellet intact. Gently flick tube to disperse cell pellet.
Remove Geltrex from the coated well.
Gently resuspend human iPSC pellet in an appropriate volume of STEMPRO. Distribute between wells of feeders, depending on the proliferation rate). Human iPSCs can be passaged in a split ratio of 1:2 to 1:6.
Carefully place into 5% CO2 incubator, swirl the plate carefully to ensure an even distribution of cells across the wells.
Feed cells daily until cells are ready to be split again (when cells reach 80% confluency).
Passage human iPSCs onto new Geltrex-coated well (step 1.3 to 1.9) in a split ratio of 1:2. Small colonies should be formed and distributed evenly on Geltrex-coated well prior to transfection.
2. Transfection of human iPSCs with GeneJuice
Cells (grown on 6-well plates) should be approximately 40 -50% confluent on the day of transfection to achieve optimal transfection efficiency. It is not necessary to change the cell medium until the next day.
Prepare 100 μl KO-DMEM/F12 in a sterile 1.5 ml eppendorf tube. Add 27 μl GeneJuice transfection reagent. Mix well. Incubate at room temperature for 5 mins.
Add 4 μg plasmid DNA. Incubate the tube at room temperature for 15 mins. The choice of plasmid is critical for optimal transfection efficiency. We use a plasmid with an enhanced green fluorescence protein (eGFP) driven by CAG promoter5 (pCAG-eGFP). CAG promoter is a strong promoter that is transcriptionally active in human iPSCs and thus can be used to drive transgene expression in these cells. In our hands, linearization of plasmid did not seem to affect transfection efficiency.
Add GeneJuice-DNA mixture to the cells and swirl the plate. Spin the plate at 1200 rpm for 5 minutes ('spinoculation' method) to increase the contact of transfection mixture with human iPSCs on the well.
Incubate the cells at 37°C overnight.
Monitor for transfection efficiency the next day.
For stable transfection, change medium the next day with fresh STEMPRO. Add appropriate antibiotic selection after 24 - 48 hours.
3. Nucleofection of human iPSCs
Human iPSCs grown on feeders or Geltrex can be used for nucleofection. However, we strongly recommend replating nucleofected human iPSCs onto mouse embryonic fibroblast (MEF) or human foreskin fibroblast (HFF) feeders to ensure high cell viability and recovery. At least 2 million cells should be used to achieve higher cell survival following nucleofection.
Prepare feeder-cell conditioned media (CM) by incubating human iPSC KnockOut Serum Replacement (KOSR) media with feeder cells overnight. Collect CM every 24 hours.
Note: Any mouse strain used for preparing feeder layers (such as BLK6, CF1 and MF1) is suitable to be used for making CM.
On the day of nucleofection, pre-treat human iPSCs with 10 μM ROCK inhibitor for at least 1 hour.
Prepare 82 μl of human stem cell nucleofector solution in a sterile 1.5 ml eppendorf tube. Add 18 μl supplement 1. Mix well. Incubate solution at 37°C for 5 mins.
Pre-warm feeder cell-conditioned media (CM) and 0.25% trypsin/ EDTA to 37°C. Under the hood, prelabel one sterile 15 ml conical tube.
Carefully remove media from human iPSC culture to be nucleofected. Gently wash the cells with 2 ml 1X phosphate buffered solution (PBS) per well. Aspirate the PBS. Add 1 ml of 0.25% trypsin per well. Incubate cells at 37°C for 3 mins.
Gently triturate the cells with 1000 ml pipette tip. Wash the bottom of the well to make sure all human iPSCs are completely detached. Transfer cell suspension to the labeled 15 ml conical tube.
Note: Trypsinization into single cells should strictly be avoided. Cells should only be dislodged into small clumps of cells consisted of approximately triplets of cells. Small clumps of cells (cell triples) will be dissociated into single cells during the subsequent handling of the cells.
Add 9 ml MEF media to inactivate trypsin. Spin cells at 800 rpm for 3 mins. Carefully aspirate the supernatant and leave the cell pellet intact.
Resuspend cells in prewarmed 100 μl of human stem cell nucleofector solution from Step 3.3.
Transfer cells to a nucleofector cuvette using a 1 ml pipette tip.
Add 4 μg of plasmid DNA into the cell suspension in the cuvette. Mix cells and DNA by gentle swirling. Tap the cuvette twice on the hood surface.
Insert the cuvette into the nucleofector holder. Use programs B-016. Nucleofect cells by pressing button X.
<> will be displayed when the nucleofection process is completed (usually only takes 1-5 seconds). The use of other nucleofection programs (A-023, A-033 and U-023) yielded less than 10% of eGFP-positive cells from human iPSCs.
Retrieve nucleofected cells from the cuvette using the provided Pasteur plastic pipette. Recover cells by resuspending them in prewarmed CM and ROCK inhibitor into a sterile 1.5 ml eppendorf tube. Incubate cells at 37°C for 10 mins to let cells recover.
Transfer cells drop-wise onto feeder layers using 1 ml pipette tip. Incubate the cells at 37°C overnight.
Monitor for transfection efficiency the next day. To derive stable clones of transgenic human iPSCs that stably expressing transgene, change medium the next day with CM and ROCK inhibitor. Add appropriate antibiotic selection after 24 - 48 hours.
4. Representative Results:
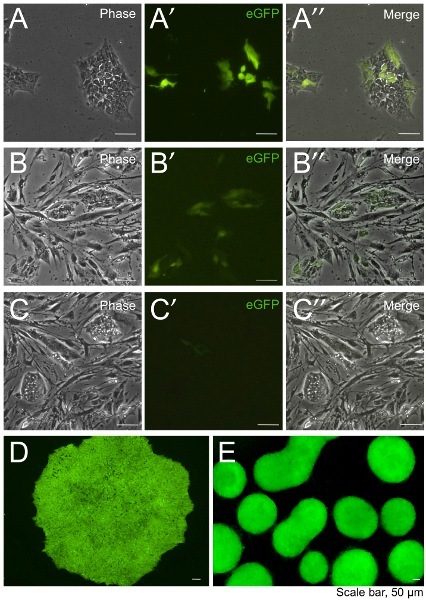 Figure 1. Photomicrographs of Riv1 human iPSCs transfected with pCAG-eGFP. (A) eGFP-expressing Riv1 cells transfected using GeneJuice on Geltrex 12 hours post-transfection. Colonies of Riv1 human iPSCs plated and formed on feeders following nucleofection using B-016 (B) and A-023 (C) program. (D) Stably eGFP-expressing human iPSC colonies with ubiquitous eGFP expression derived from GeneJuice. (E) Stably-transfected Riv1 human iPSCs retained constitutive eGFP expression during embryoid body differentiation.
Figure 1. Photomicrographs of Riv1 human iPSCs transfected with pCAG-eGFP. (A) eGFP-expressing Riv1 cells transfected using GeneJuice on Geltrex 12 hours post-transfection. Colonies of Riv1 human iPSCs plated and formed on feeders following nucleofection using B-016 (B) and A-023 (C) program. (D) Stably eGFP-expressing human iPSC colonies with ubiquitous eGFP expression derived from GeneJuice. (E) Stably-transfected Riv1 human iPSCs retained constitutive eGFP expression during embryoid body differentiation.
Discussion
Our protocols result in simple, robust and highly reproducible techniques to introduce transgenes into human iPSCs without prominent toxic effect and cell death. Human iPSCs should be passaged into smaller clumps of cells (5-10 cells) and plated on Geltrex at high density (1:2) to ensure optimal transfection efficiency in numerous small colonies. For human iPSC lines that are more prone to differentiation and cell death, higher number of human iPSCs (up to 4 X 106 cells) should be used for a single nucleofection experiment. Transient transfection assay generates large numbers of transgene-expressing human iPSCs within 1 day. Stably transfected iPSC clones usually appear within 7 days, and these transgenic colonies should be ready to be picked within three weeks. The use of CAG promoter described here ensures ubiquitous expression of eGFP reporter. Under such improvements, our protocols can be fed into other applications, including overexpression, conditional induction, derivation of lineage-specific reporter lines, shRNA or siRNA knockdown, gene targeting and homologous recombination.
Disclosures
No conflicts of interest declared.
Acknowledgments
Work described in this manuscript was made possible by funding from the California Institute for Regenerative Medicine (CIRM) for UCR Stem Cell Core.
References
- Liew CG. Human embryonic stem cells: possibilities for human cell transplantation. Ann Med. 2005;37:521–532. doi: 10.1080/07853890500379463. [DOI] [PubMed] [Google Scholar]
- Eiges R. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. Homogous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- Siemen H. Nucleofection of human embryonic stem cells. Stem Cells Dev. 2005;14:378–383. doi: 10.1089/scd.2005.14.378. [DOI] [PubMed] [Google Scholar]
- Liew CG, Draper JS, Walsh J, Moore H, Andrews PW. Transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25:1521–1528. doi: 10.1634/stemcells.2006-0634. [DOI] [PubMed] [Google Scholar]
- Xia X, Ayala M, Thiede BR, Zhang SC. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells. 2008;26:525–533. doi: 10.1634/stemcells.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam SR. Improved genetic manipulation of human embryonic stem cells. Nat Methods. 2008;5:389–392. doi: 10.1038/nmeth.1200. [DOI] [PubMed] [Google Scholar]
- Braam SR. Feeder-free culture of human embryonic stem cells in conditioned medium for efficient genetic modification. Nat Protoc. 2008;3:1435–1443. doi: 10.1038/nprot.2008.140. [DOI] [PubMed] [Google Scholar]
- Hohenstein KA, Pyle AD, Chern JY, Lock LF, Donovan PJ. Nucleofection mediates high-efficiency stable gene knockdown and transgene expression in human embryonic stem cells. Stem Cells. 2008;26:1436–1443. doi: 10.1634/stemcells.2007-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placantonakis DG. BAC transgenesis in human embryonic stem cells as a novel tool to define the human neural lineage. Stem Cells. 2009;27:521–532. doi: 10.1634/stemcells.2008-0884. [DOI] [PubMed] [Google Scholar]


