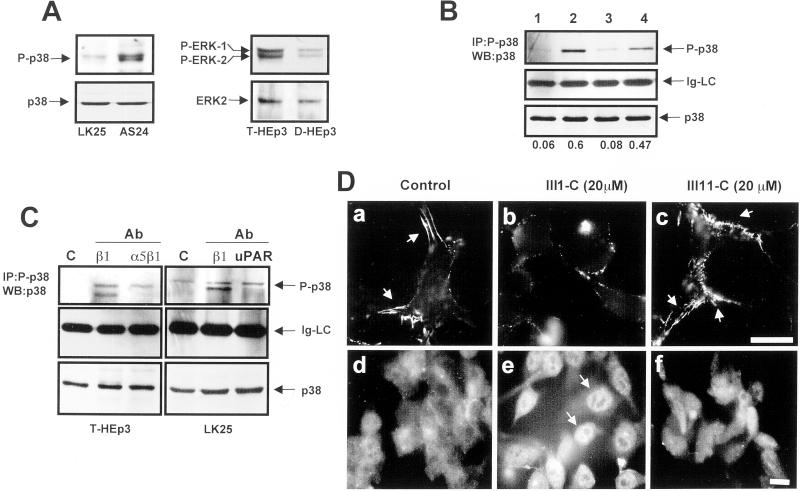
Figure 3.
Disruption of FN fibrillogenesis alters the p38/ERK activity ratio. (A) Basal levels of active and total p38 in LK25 and AS24 cells, serum-starved for 24 h (left) or basal levels of active and total ERK in T-HEp3 and D-HEp3 cells serum-starved for 24 h (right) were detected by Western blotting using anti-phospho-p38 (P-p38) and p38 antibodies (p38) or anti-phospho-ERK (P-ERK1/2) and anti-ERK (ERK2) antibodies. (B) Active p38 in T-HEp3 (lane 1), D-HEp3 (lane2), LK25 (lane3), or AS24 (lane 4) cells was detected by IP with anti-phospho-p38 antibodies and blotting with anti-p38 antibodies (top, P-p38). Middle, light chain of IgG (Ig-LC), as a gel-loading control. Bottom (p38), total p38 in cell lysates used for IP as detected by Western blotting. The numbers below each lane show the P-p38 to p38 ratio. (C) Effect of blocking FN fibrillogenesis or disruption of uPAR-integrin complex on p38 activation. Cells were plated in gelatin-coated dishes and treated with anti-β1 or anti-α5β1 antibodies as indicated in Figure 2A (T-HEp3, left) or with anti-β1 and anti-uPAR antibodies (LK25, right). The cells were lysed and active p38 was detected as in B. c, control (no antibody); cells treated with antibodies to: β1 (AIIB2, 10 μg/ml), α5β1 (BIIG2, 20 μg/ml), or uPAR (R2, 10 μg/ml). Control LK25 cells always displayed a slightly higher level of active p38 than T-HEp3 cells (see B and C). (D) Effect of FN fibrils disruption with FN III1-C fragment on p38 activation. LK25 cells were plated in FN-depleted FBS supplemented with 5 μg/ml human FN. After overnight incubation to allow for FN fibrillogenesis, the cells were left untreated (control, a and d) or were treated for 16 h with 20 μM III1-C (b and e) or III11-C (c and f) fragments of FN. Cells were then fixed and stained for either FN (a–c) or with anti-p38 monoclonal antibodies (d–f) and visualized by standard IF. The arrows indicate FN fibrils present only in untreated or control (III11-C) -treated cells (a and c) or p38 in the nucleus (e) after FN fibrils disruption with III1-C FN fragment. Bars, 40 μm.