Abstract
Background:
E-cadherin is known to be an invasion suppressor gene and cathepsin-D, a protease, which is an invasion promoter and plays a central role in solid tumors including oral cancer.
Aims:
To look for the expression pattern in normal buccal mucosa, dysplastic oral epithelium and oral squamous cell carcinoma (SCC) along with their correlation to individual atypical features, thereby providing an objective to the grading system in predicting the fate of affected epithelium.
Materials and Methods:
To elucidate the expression patterns of these markers, we examined immunohistochemically on formalin fixed, paraffin embedded sections 22 dysplastic epithelia, eight SCC and ten normal buccal mucosa.
Results:
In dysplastic epithelium slight loss of expression of E-cadherin was noted as grade of dysplasia increased. Two cases of carcinoma clearance showed only basal and suprabasal staining. The staining varied in SCC with patchy to complete absence of expression. With cathepsin-D fine to moderate granular cytoplasmic staining was noted in most of the dysplastic epithelium. Similar staining was noted in SCC. The atypical features which strongly correlated to loss of expression of E-cadherin and intense cathepsin-D expression are basilar hyperplasia, loss of cohesion, mitosis, loss of polarity and drop shaped rete ridges.
Conclusions:
The result of the study shows that the above atypical features should be given more weightage in addition to traditional grading system, in predicting the fate of affected epithelium. Additional studies with larger sample size and using monoclonal antibody against cathepsin-D may further strengthen our findings.
Keywords: Cathepsin-D, dysplastic epithelium, E-cadherin, immunohistochemistry, normal oral mucosa, oral squamous cell carcinoma
INTRODUCTION
Carcinogenesis is a multi-stage process which requires modulation of cell-cell and cell-stromal interactions.[1,2] E-cadherins are important transmembrane glycoproteins that are prime mediators of cell-cell adhesion[3] and its loss enhances the invasion.[4] Loss of E-cadherin expression has been noted with poorly differentiated morphology in a large number of malignancies such as cervix, esophagus, including head and neck.[5,6] Cathepsin-D is an aspartic lysosomal enzyme which proteolytically degrades the extracellular matrix and proteoglycans.[7–9] Loss of E-cadherin and increased cathepsin-D expression is observed in carcinoma which helps in metastasis. Taking these features into consideration we looked at their expression pattern in premalignant lesions/dysplastic oral epithelium. The rationale of the study is not to establish cathepsin-D and E-cadherin as a marker for premalignant lesions, but to correlate their expression with features seen in hematoxylin and eosin stained sections, as these features can acquire invasive property, there by predicting the fate of affected epithelium in terms of potential for malignant transformation.
MATERIALS AND METHODS
The materials for the present study comprised a total of 40 biopsy specimens retrieved from the files of Department of Oral Pathology and Microbiology, The Oxford Dental College, Hospital and Research Centre, Bangalore. The archival material retrieved comprised of 22 cases of oral precancerous/dysplastic epithelium and the criteria employed in the present study was all precancerous epithelium showing some grade of dysplasia with or without surface keratosis in all cases (including four cases of carcinoma clear margin and one case of dysplastic epithelium with adjacent infiltrating carcinoma), eight cases of oral squamous cell carcinoma (SCC) and ten biopsies were obtained from normal buccal mucosa.
Antibodies used (DAKO cytomation)
Monoclonal mouse antihuman E-cadherin primary antibody, 0.2 ml was diluted before use by adding 20μl of antibody to 980μl of TBS of pH 7.4. (Clone NCH-38, M3612).
Polyclonal rabbit anti-human primary cathepsin-D, 7ml ready to use (N1625).
Biotinylated anti-mouse, anti-rabbit, Igs, LINK/secondary antibody. (LSAB+ system, K0679).
Streptavidin conjugated to horseradish peroxidase. (LSAB+ system, K0679).
Liquid Diamino benzidine chromogen (DAB).
Methods
Formalin fixed, paraffin embedded sections of the normal, dysplastic epithelium and SCC was stained by hematoxylin and eosin, the serial sections of the same was stained by immunohistochemical reagents using avidin-biotin complex method. All the antibodies in the procedure were incubated at room temperature, in a moist chamber.
Procedure
The sections of 5μ thickness were cut and mounted on the 3-aminopropyl triethoxy silane coated glass slides, deparaffinized in xylene 2 changes for 5 minutes each and then the slides were hydrated through different grades of isopropyl alcohol [100% - 50% for 3 minutes each] and brought to distilled water. The slides were then antigen retrieved using citrate buffer [pH-6.0] in a pressure cooker, for two whistles and then the solution is allowed to cool to room temperature and then washed in tris buffered saline (TBS) (pH 7.2-7.6) for two changes 3 minutes each. For cathepsin-D antigen retrieved using citrate buffer [pH-6.0] in a kitchen microwave oven at temperature 600watts for 5 minutes followed by 450 watts 10 minutes and then the solution is allowed to cool to room temperature and then washed in TBS, for two changes 3 minutes each. The slides were then incubated with 3% H2O2 for 20 minutes at room temperature to block the endogenous peroxidase activity and then washed in TBS two changes for 3 minutes followed by 2% skimmed milk in TBS for 30-40 minutes to block the non-specific staining.
The slides were then incubated with monoclonal mouse antihuman E-cadherin primary antibody for 90 minutes and cathepsin-D for 30 minutes. After incubation with primary antibody sections were washed in TBS for two changes 3 minutes each and then incubated with LINK/secondary antibody for 30 minutes. After this labeling sections were washed in TBS two changes 3 minutes each and incubated with Streptavidine horse radish peroxidase for 30 minutes. This step was followed by TBS wash and then incubated with DAB chromogen, which is freshly prepared, for 8-10 minutes for E-cadherin and for 1minute in case of cathepsin-D (DAB chromogen was prepared by adding 20 μl of DAB to 1 ml of buffer substrate).
Chromogen reaction was terminated by washing in TBS and then washed in distilled water, counterstained using Mayer's hematoxylin for 4-5 minutes and then blued in running tap water for 8-10 minutes. The slides were then dehydrated using graded isopropyl alcohol (50%- 100%) and then cleared using xylene changes for 10 minutes and mounted in DPX and viewed under binocular light microscope.
The expression pattern of E-cadherin and cathepsin-D was studied in the entire length of the epithelium in normal, dysplastic epithelium and in carcinoma along with its preceding serial sections stained with hematoxylin and eosin. The loss of E-cadherin was taken into consideration in the dysplastic epithelium and the corresponding hematoxylin and eosin sections was reviewed for individual atypical features corresponding to loss of expression of E-cadherin in IHC slide. Similarly intense/marked staining for cathepsin-D was looked in the dysplastic epithelium with its correlation to atypical features in hematoxylin and eosin. The number of cases in which loss of E-cadherin and intense/marked cathepsin-D staining corresponding to particular atypical morphologic features seen in the hematoxylin and eosin section was noted. The sensitivity of these features was then statistically analyzed.
To further validate the findings and to look for prognostic value of these atypical features, we also studied at the islands and surface epithelium of eight carcinoma cases, by correlating the loss of expression of E-cadherin and intense expression of cathepsin-D to atypical features seen in hematoxylin and eosin sections. A comparative study of expression pattern of E-cadherin and cathepsin-D was made between normal, dysplastic epithelium and in carcinoma.
Statistical methods
Sensitivity and the correlation by Goodman-Kruskal Gamma method has been used to find the correlation of loss of expression of E-cadherin and Intense/marked staining of cathepsin-D with respect to 12 atypical morphologic features of hematoxylin and eosin.
Goodman–Kruskal Gamma correlation co-efficient: Up to 0.1 Trivial correlation, 0.1-0.3 Small correlation, 0.3-0.5 Moderate correlation, 0.5-0.7 Large correlation, 0.7-0.9 Very large correlation, 0.9-1.0 Nearly perfect correlation, 1.0 Perfect correlation.
OBSERVATION AND RESULTS
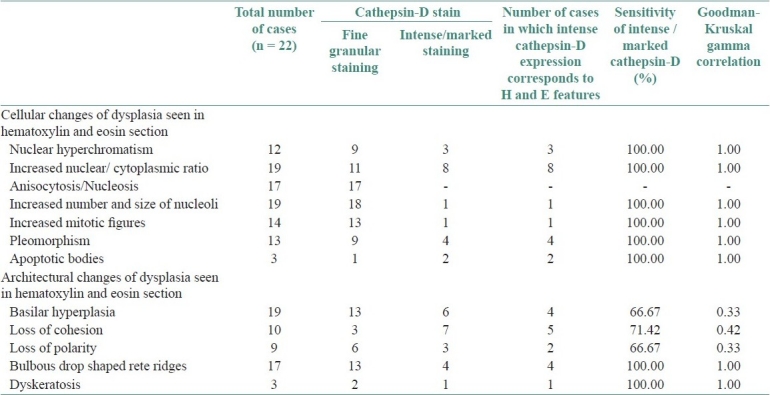
Of the 12 atypical morphologic features studied none of the cases showed irregular epithelial stratification and bizarre mitosis. The highest sensitivity [100%] of loss of expression of E-cadherin was seen in increased nucleo-cytoplasmic ratio, mitosis, level of mitosis restricted to lower half of the epithelium, with a perfect correlation [1.00]. Pleomorphism showed very large correlation [0.78] with a very good sensitivity of 88.88%. There was 80% sensitivity in relation to loss of polarity and prominent nucleoli with large correlation [0.5-0.7]. There was moderate correlation [0.3-0.5] with loss of cohesion, basilar hyperplasia, nuclear hyperchromatism, but showed a good sensitivity for the loss of expression of E-cadherin. There was small and no correlation to drop shaped reteridges and intra epithelial keratinization respectively. Table 1 shows atypical features seen in hematoxylin and eosin section and the number of cases in which loss of E-cadherin expression corresponding to atypical features along with their sensitivity and correlation coefficient. Similarly intense /marked cathepsin-D expression with high sensitivity and perfect correlation was seen in relation to drop shaped reteridges, intra epithelial keratinization, nuclear hyperchromatism, prominent nucleoli, increased nucleo cytoplasmic ratio, pleomorphism, mitosis. There was moderate correlation with loss of cohesion, basilar hyperplasia, and loss of polarity, with a sensitivity of 71.42%, 66.67% and 66.67% respectively. Table 2 shows atypical features seen in hematoxylin and eosin section and the number of cases in which intense/marked expression of cathepsin-D corresponding to atypical features along with their sensitivity and correlation coefficient.
Table 1.
Correlation of loss of E-cadherin with atypical morphologic features
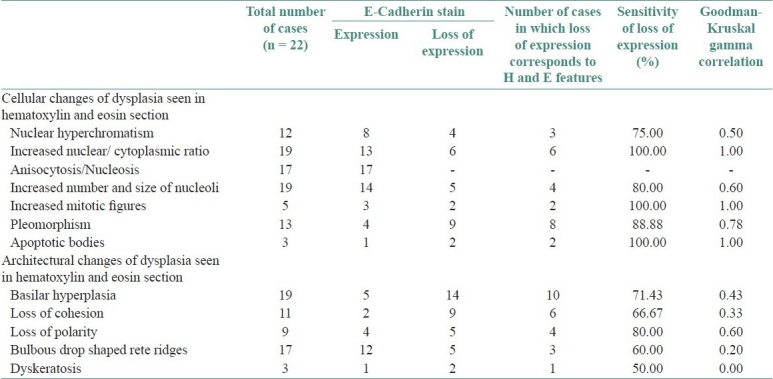
Table 2.
Correlation of cathepsin-D with atypical morphologic features
E-cadherin expression in normal buccal mucosa
Normal epithelium displayed the pattern of staining which was present in a pericellular distribution throughout the basal, suprabasal and prickle cell layers. Staining was weak in the basal aspect of keratinocytes in one case and was absent in the superficial layers in all cases. Although the pattern of staining was same in all cases examined, the staining varied in intensity in some cases.
E-cadherin expression in the dysplastic epithelium
Of the 22 dysplastic epithelium the expression pattern was similar to normal mucosa in all mild dysplasia with absence of staining in granular layer, but slight loss of expression pattern was noted as the severity of dysplasia increased. One case showed full length epithelial staining which was atrophic epithelium and two cases of carcinoma clear margin showed only basal and suprabasal staining. No cases showed complete loss of expression. Weak expression was noted in the area of intracellular edema. Loss of expression of E-cadherin was associated with inflammatory infiltrate in the epithelium.
E-cadherin expression in oral SCC
All the eight cases of SCC were well to moderately differentiated (2 well and six moderately differentiated carcinomas). All the tumors showed reduced and heterogeneous staining. No tumor was completely negative for E-cadherin distribution. Many small islands and single infiltrating epithelial cells showed negative to patchy staining. The pericellular E-cadherin expression was fragmented with loss of staining coinciding with loss of cellular adhesion, separation of tumor cells and keratinization. The overlying epithelium in one case showed loss of expression in the prickle cell layer.
Cathepsin-D expression in normal buccal mucosa
Cathepsin-D in normal epithelium stained as a fine granular pattern in the basal layer than in other layers. Two cases showed a homogeneous granular staining pattern in the cytoplasm of the entire length of the epithelium with slight intense staining of superficial layer. More granular staining was seen at the periphery of the tissue in all cases possibly due to edge effect or fringe effect.
Cathepsin-D expression in the dysplastic epithelium
The distribution of cathepsin-D was distinct in all precancerous/dysplastic epithelium. There was almost fine to moderate paranuclear granular staining pattern with in the cytoplasm of entire length of epithelium. There was almost moderate to intense staining of granular and superficial layers. A few cases showed only fine granular staining as seen in normal mucosa with slight more staining in the basal layers. There was no change in the staining intensity in carcinoma clear margin cases from that of other keratotic lesions. The underlying inflammatory cells and macrophages showed intense staining in all cases.
Cathepsin-D expression in oral SCC
Two staining patterns were evident, a cytoplasmic granular pattern that was intense and both granular and densely diffuse staining appeared in one case. The infiltrating tumor cells were strongly positive in three cases compared to others. Both paranuclear punctuate, diffuse cytoplasmic and cell surface immune staining was noted. Some tumor islands showed strong staining of keratin pearls.
DISCUSSION
Several attempts and many criteria have been suggested for the diagnosis of dysplastic grades or cytologic changes from the basal cell layer and upward.[10–13] Expression of thousands of genes during tumor progression by array technologies which may form a basis for a detailed molecular characterization of cancer development.[14,15] A recent impressive series of studies has focused on DNA ploidy measurements in patients with oral epithelial dysplasia during a rather long follow-up period.[16–18] It has been reported that E-cadherin was variable in severe dysplasia without adjacent carcinoma and also its complete loss from carcinoma-in-situ adjacent to infiltrating carcinoma supports the invasion suppressor function of E-cadherin.[19]
In our study the E-cadherin expression in normal buccal mucosa was extended to the superficial compartment and absent in the superficial layers, which suggests that its absence may helps in normal desquamation similar to that seen in cervical squamous epithelium.[13] Differences in the expression of E-cadherin in the dysplastic epithelium with varying degrees of dysplasia or severity of dysplasia and area of tissue, would suggest that these alterations in E-cadherin expression is the result of the progression of dysplasia and could be a late event changing towards a cell phenotype with the ability to invade. To our best knowledge no literature correlates expression of E-cadherin and cathepsin-D to atypical features neither in oral epithelial dysplasia nor in dysplasia in other areas of the body. In our study most common atypical features observed in hematoxylin and eosin section with loss of expression of E-cadherin was in relation to loss of cohesion, apoptotic bodies and mitosis [Figures 1a–b and 2], basilar hyperplasia, nuclear hyperchromatism [Figure 3a–b] and intense /marked cathepsin-D expression was in relation to basilar hyperplasia, loss of polarity [Figure 4a–b], loss of cohesion and mitosis [Figures 5 and 6], increased nucleo-cytoplasmic ratio, pleomorphism with a good sensitivity and Goodman-Kruskal Gamma correlation co-efficient. But when comparing the homogeneity or statistical similarity of loss of expression of E-cadherin between dysplastic and carcinomatous epithelium the following features were found to be more significant: Pleomorphism, basilar hyperplasia, nuclear hyperchromatism, loss of polarity, mitosis and loss of cohesion.
Figure 1.
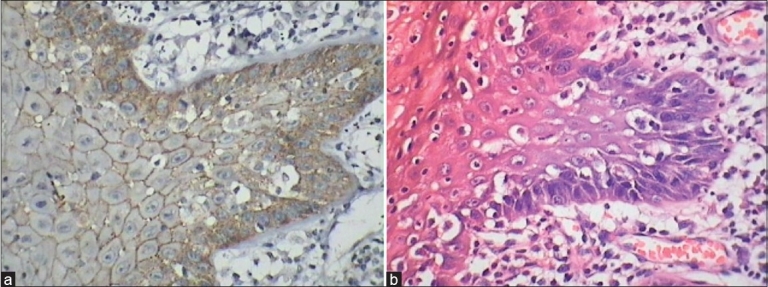
(a) Loss of E-cadherin in relation to loss of cohesion, apoptotic bodies (b) H & E showing loss of cohesion and apoptosis, mitosis
Figure 2.
Loss of E-cadherin expression in relation to mitosis
Figure 3.
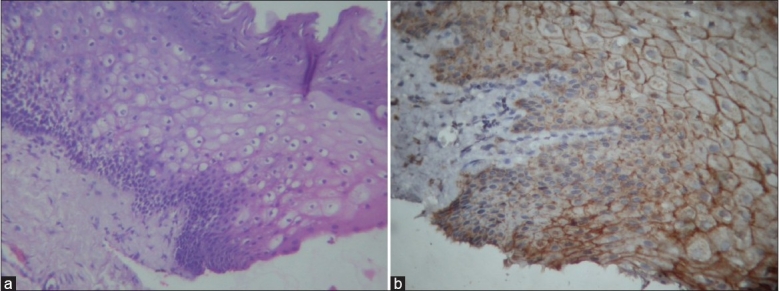
(a) Loss of E-cadherin in relation to basilar hyperplasia and nuclear hyperchromatism. 40× (b) IHC slide showing loss of E-cadherin in relation to basilar hyperplasia and nuclear hyperchromatism. 40×
Figure 4.
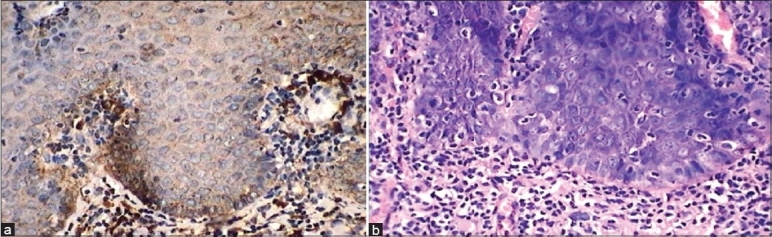
(a) IHC shows intense/marked cathepsin-D expression in relation to basilar hyperplasia and loss of polarity. 40× (b) H and E shows Intense/marked cathepsin-D expression in relation to basilar hyperplasia and loss of polarity. 40×
Figure 5.
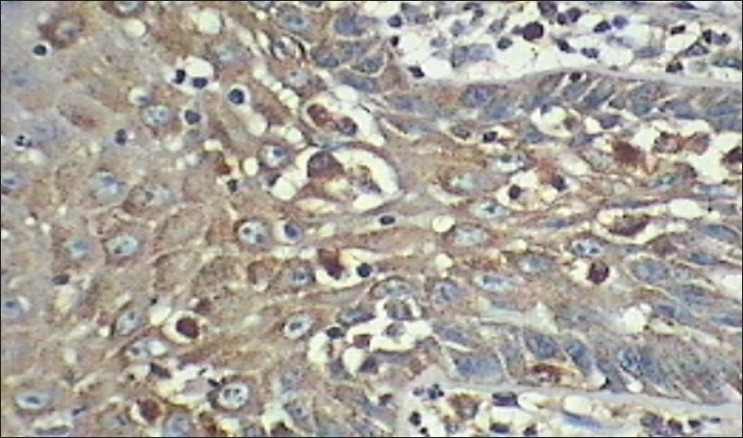
Intense cathepsin-D expression in loss of cohesion, apoptotic bodies and mitosis 40×
Figure 6.
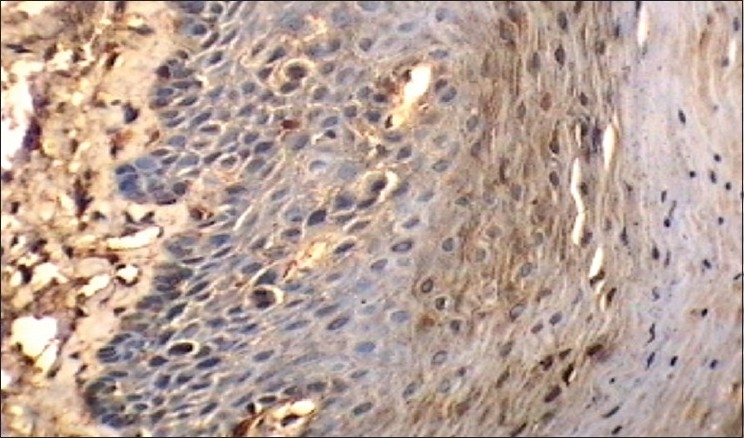
Intense/marked cathepsin-D in relation to mitosis
Cathepsin-D is a major protein-degrading enzyme and is required in certain epithelial tissue for tissue remodeling and renewal and acts like mitogen.[20,21] As cathepsin-D is an ubiquitous enzyme present in many normal tissues[7] but we didn’t come across any literature of cathepsin-D expression in oral mucosa and with our observation, which showed as a fine granular cytoplasmic staining, it may serve a similar function to that of other tissue.[7,21,22] Studies have suggested that extracellular matrix invasion is not merely caused by passive growth pressure and that invasive tumor cell require degradative enzymes against both collagenous and non-collagenous components of extracellular matrix and several proteinases have been implicated including cathepsin-D.[23]
Loss of E-cadherin has the ability to change its cell phenotype.[24,25] In view of this, a single transformed cell whether in the basal layer or in the any length of the epithelium can acquire invasive property. However immunohistochemical determination of protease levels is less sensitive, less accurate, and more subjective than the enzymatic determination from tumor homogenates,[20] our study also observed a similar result in normal epithelium, dysplastic epithelium, SCC, fibroblasts and inflammatory cells, which all expressed the cathepsin-D.
In our study most of the dysplastic epithelium showed moderate to intense staining, especially at the granular and superficial layers, which may indicate its function in maturative process, as the epithelium is differentiated. When comparing the homogeneity or statistical similarity of intense staining of cathepsin-D between dysplastic epithelium and SCC the following features were found to be more significant: drop shaped reteridges, intraepithelial keratinization, basilar hyperplasia, loss of cohesion, loss of polarity and mitosis. So by considering the statistical results of both loss of E-cadherin and intense cathepsin-D, the individual atypical morphologic features which are given more importance are: basilar hyperplasia, drop shaped reteridges, loss of cohesion, loss of polarity and mitosis.
In conclusion, incorporating immunohistochemical analysis of E-cadherin and cathepsin-D, which are documented indicators of invasive propensity and corresponding with H and E sections, the following features show a strong correlation with invasive potential: basilar hyperplasia, drop shaped rete ridges, loss of cohesion, loss of polarity and mitosis. These findings suggests that in addition to conventional grading, additional weightage should be given to the above mentioned atypical features and epithelia exhibiting these features whether qualifying conventionally to fit any of the grades of dysplasia or not, should be viewed with greater caution and handled appropriately. Additional studies with larger sample size and using monoclonal antibody against cathepsin-D may further strengthen our findings. As is to be expected no one biomarker appears to be of outstanding merit. An integration of results may eventually yield information of improved predictive value than the standard histopathologic parameters. Loss of E-cadherin and increased cathepsin-D expression are at greater risk of invasive transformation in many premalignant lesions and tumors of the body. Since there is intra and inter observer variability in grading the dysplasia we made an attempt to predict the fate of affected epithelium in terms of malignant transformation as the dysplastic epithelium (atypia) showing loss of E-cadherin and increased cathepsin-D can be given more importance than conventional grading system as these features can acquire invasive property.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lo Muzio L, Pannone G, Leonardi R, Staibano S, Mignogna MD, De Rosa G, et al. Survivin, a potential early predictor of tumor progression in the oral mucosa. J Dent Res. 2003;82:923–8. doi: 10.1177/154405910308201115. [DOI] [PubMed] [Google Scholar]
- 2.Yonemura Y, Nojima N, Kaji M, Fujimura T, Itoh H, Ninomiya I, et al. E-cadherin and urokinase-type plasminogen activator tissue status in gastric carcinoma. Cancer. 1995;76:941–53. doi: 10.1002/1097-0142(19950915)76:6<941::aid-cncr2820760606>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Mareel M, Leroy A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol Rev. 2002;83:337–76. doi: 10.1152/physrev.00024.2002. [DOI] [PubMed] [Google Scholar]
- 4.Wijnhoven BPL, Dinjens WNM, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith MEF, Pignatelli M. The molecular histology of neoplasia: the role of the cadherin/catenin complex. Histopathology. 1997;31:107–11. doi: 10.1046/j.1365-2559.1997.2350845.x. [DOI] [PubMed] [Google Scholar]
- 6.Vessey CJ, Wilding J, Folarin N, Hirano S, Takeichi M, Soutter P, et al. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J Pathol. 1995;176:151–9. doi: 10.1002/path.1711760208. [DOI] [PubMed] [Google Scholar]
- 7.Reid WA, Valler MJ, Kay J. Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol. 1986;39:1323–30. doi: 10.1136/jcp.39.12.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandour-Edwards R, Trock B, Donald PJ. Predictive value of cathepsin-D for cervical lymph node metastasis in head and neck squamous cell carcinoma. Head Neck. 1999;21:718–22. doi: 10.1002/(sici)1097-0347(199912)21:8<718::aid-hed6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Ikeguchi M, Sakatani T, Ueta T, Fukuda K, Oka S, Hisamitsu K, et al. Correlation between cathepsin D expression and p53 protein nuclear accumulation in oesophageal squamous cell carcinoma. J Clin Pathol. 2002;55:121–6. doi: 10.1136/jcp.55.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CJ, Pindborg JJ. Histologic grading of oral epithelial dysplasia by the use of photographic standards. Copenhagen: WHO reference centre for oral precancerous conditions; 1969. [Google Scholar]
- 11.Bancoczy J, Csiba A. Occurrence of epithelial dysplasia in oral leukoplakia-Analysis and follow up study of 120 cases. Oral Surg Oral Med Oral Patho. 1976;42:766–74. doi: 10.1016/0030-4220(76)90099-2. [DOI] [PubMed] [Google Scholar]
- 12.Lumermann H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive SCC. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:321–9. doi: 10.1016/s1079-2104(05)80226-4. [DOI] [PubMed] [Google Scholar]
- 13.Speight P, Farthing PM, Bouquot JE. The pathology of oral cancer and precancer. Curr Diagn Pathol. 1996;3:165–77. [Google Scholar]
- 14.Patel V, Leethanakul C, Gutkind JS. New approaches to the understanding of the molecular basis of oral cancer. Crit Rev Oral Biol Med. 2001;12:55–63. doi: 10.1177/10454411010120010401. [DOI] [PubMed] [Google Scholar]
- 15.Thykjaer T, Workman C, Kruhøffer M, Demtröder K, Wolf H, Andersen LD, et al. Identification of gene expression patterns in superficial and invasive human bladder cancer. Cancer Res. 2001;61:2492–9. [PubMed] [Google Scholar]
- 16.Sudbo J, Ried T, Bryne M, Kildal W, Danielsen H, Reith A. Abnormal DNA content predicts the occurrence of carcinomas in non-dysplastic oral white patches. Oral Oncol. 2001;37:558–65. doi: 10.1016/s1368-8375(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 17.Sudbø J, Kildal W, Risberg B, Koppang HS, Danielsen HE, Reith A. DNA content as a prognostic marker in patients with oral leukoplakia. N Engl J Med. 2001;344:1270–8. doi: 10.1056/NEJM200104263441702. [DOI] [PubMed] [Google Scholar]
- 18.Sudbø J, Kildal W, Johannessen AC, Koppang HS, Sudbø A, Danielsen HE, et al. Gross genomic aberrations in precancers: clinical implications of a long-term follow-up study in oral erythroplakias. J Clin Oncol. 2002;20:456–62. doi: 10.1200/JCO.2002.20.2.456. [DOI] [PubMed] [Google Scholar]
- 19.Williams HK, Sanders DS, Jankowski JA, Landini G, Brown AM. Expression of cadherins and catenins in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 1998;27:308–17. doi: 10.1111/j.1600-0714.1998.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 20.Rochefort H, Liaudet-Coopman E. Cathepsin-D in cancer metastasis: a protease and a ligand. APIMS. 1999;107:86–95. doi: 10.1111/j.1699-0463.1999.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 21.Marsigliante S, Biscozzo L, Resta L, Leo G, Mottaghi A, Maiorano E, et al. Immunohistochemical and immunoradiometric evaluation of total cathepsin-D in human larynx. Eur J Cancer Oral Oncol. 1994;30:51–5. doi: 10.1016/0964-1955(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 22.Horikoshi T, Arany I, Rajaraman S, Chen SH, Brysk H, Lei G, et al. Isoforms of cathepsin D and human epidermal differentiation. Biochime. 1998;80:605–12. doi: 10.1016/s0300-9084(98)80013-8. [DOI] [PubMed] [Google Scholar]
- 23.Castiglioni T, Merino MJ, Elsner B, Lah TT, Sloane BF, Emmert-Buck MR. Immunohistochemical analysis of cathepsin D, B and L in human breast cancer. Hum Path. 1994;25:857–62. doi: 10.1016/0046-8177(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 24.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: Epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–47. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen H, Mejlvang J, Mahmood S, Gromova I, Gromov P, Lukanidin E, et al. Immediate and delayed effects of E-cadherin inhibition on gene regulation and cell motility in human epidermoid carcinoma cells. Mol Cell Biol. 2005;25:9138–50. doi: 10.1128/MCB.25.20.9138-9150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


