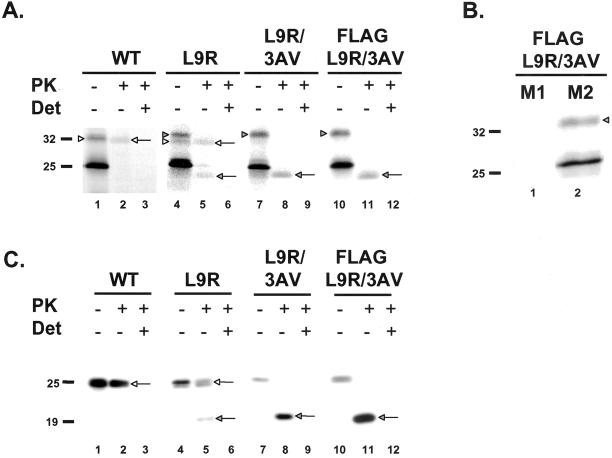
Figure 3.
Mutations in the signal sequence increase the proportion of CtmPrP. (A) In vitro translation and PK protection assays of wild-type and mutant PrPs were performed as in Figure 2. The full-length forms of SecPrP and CtmPrP are indicated by the white and shaded arrowheads, respectively (lanes 1, 4, 7, and 10). The protease-protected forms of SecPrP and CtmPrP are indicated by the white and shaded arrows, respectively (lanes 2, 5, 8, and 11). (B) FLAG-L9R/3AV PrP was synthesized by in vitro translation, and was immunoprecipitated with anti-FLAG antibodies M1 (lane 1) or M2 (lane 2). Neither CtmPrP (shaded arrowhead) nor untranslocated/unglycosylated PrP (25 kDa) are immunoprecipitated by M1, whereas both forms are immunoprecipitated by M2. (C) BHK cells were transiently transfected with plasmids encoding wild-type or mutant PrPs. Postnuclear supernatants prepared from cells 24 h after transfection were incubated with (lanes 2, 3, 5, 6, 8, 9, 11, and 12) or without (lanes 1, 4, 7, and 10) PK in the presence (lanes 3, 6, 9, and 12) or absence (lanes 1, 2, 4, 5, 7, 8, 10, and 11) of Triton-X-100 (Det). Proteins were then solubilized in SDS, deglycosylated with PNGase F, and subjected to Western blotting with 3F4 antibody. The protease-protected forms of SecPrP and CtmPrP are indicated by the white and shaded arrows, respectively.