Abstract
Background and Aims:
Opioids as epidural adjunct to local anesthetics (LA) have been in use since long and α-2 agonists are being increasingly used for similar purpose. The present study aims at comparing the hemodynamic, sedative, and analgesia potentiating effects of epidurally administered fentanyl and dexmedetomidine when combined with ropivacaine.
Methods:
A total of one hundred patients of both gender aged 21-56 years, American Society of Anaesthesiologist (ASA) physical status I and II who underwent lower limb orthopedic surgery were enrolled into the present study. Patients were randomly divided into two groups: Ropivacaine + Dexmedetomidine (RD) and Ropivacaine + Fentanyl (RF), comprising 50 patie nts each. Inj. Ropivacaine, 15 ml of 0.75%, was administered epidurally in both the groups with addition of 1 μg/kg of dexmedetomidine in RD group and 1 μg/kg of fentanyl in RF group. Besides cardio-respiratory parameters and sedation scores, various block characteristics were also observed which included time to onset of analgesia at T10, maximum sensory analgesic level, time to complete motor blockade, time to two segmental dermatomal regressions, and time to first rescue analgesic. At the end of study, data was compiled systematically and analyzed using ANOVA with post-hoc significance, Chi-square test and Fisher's exact test. Value of P<0.05 is considered significant and P<0.001 as highly significant.
Results:
The demographic profile of patients was comparable in both the groups. Onset of sensory analgesia at T10 (7.12±2.44 vs 9.14±2.94) and establishment of complete motor blockade (18.16±4.52 vs 22.98±4.78) was significantly earlier in the RD group. Postoperative analgesia was prolonged significantly in the RD group (366.62±24.42) and consequently low dose consumption of local anaesthetic LA (76.82±14.28 vs 104.35±18.96) during epidural top-ups postoperatively. Sedation scores were much better in the RD group and highly significant on statistical comparison (P<0.001). Incidence of nausea and vomiting was significantly high in the RF group (26% and 12%), while incidence of dry mouth was significantly higher in the RD group (14%) (P<0.05).
Conclusions:
Dexmedetomidine seems to be a better alternative to fentanyl as an epidural adjuvant as it provides comparable stable hemodynamics, early onset, and establishment of sensory anesthesia, prolonged post-op analgesia, lower consumption of post-op LA for epidural analgesia, and much better sedation levels.
Keywords: Dexmedetomidine, epidural anesthesia, fentanyl, lower limb surgery, ropivacaine
INTRODUCTION
Epidural anesthesia is the most commonly used technique for providing not only peri-operative surgical anesthesia but post-op analgesia in lower abdominal and limb surgeries.[1] Early postoperative mobilization and rehabilitation with minimally associated pain and discomfort is the most desirable feature in modern orthopaedic surgery.[2–4] Many a time for achieving desired peri-operative anaesthetic effect, invariably large volumes of local anaesthetics are used, thereby increasing the possibilities of local anaesthetic toxicity and deleterious haemodynamic consequences. The new amide local anaesthetic Ropivacaine has minimal cardio-vascular and central nervous system toxicity as well as a lesser propensity of motor block during post-operative epidural analgesia.[5,6] Opioids like fentanyl have been used traditionally as an adjunct for epidural administration in combination with a lower dose of local anaesthetic to achieve the desired anaesthetic effect.[7] The addition of opioid does provide a dose sparing effect of local anaesthetic and superior analgesia but there is always a possibility of an increased incidence of pruritis, urinary retention, nausea, vomiting and respiratory depression.[8,9] Also the incidence of motor block after epidural analgesia with amide local anesthetics (LA) and opioids is approximately 4-12% which itself defeats the novel purpose of early rehabilitation.[10–12]
Dexmedetomidine is a new addition to the class of alpha-2 agonist which has got numerous beneficial effects when used through epidural route.[13] It acts on both pre and post synaptic sympathetic nerve terminal and central nervous system thereby decreasing the sympathetic outflow and nor-epinephrine release causing sedative, anti-anxiety, analgesic, sympatholytic and haemodynamic effects.[14–16] Dexmedetomidine does cause a manageable hypotension and bradycardia but the striking feature of this drug is the lack of opioid-related side effects like respiratory depression, pruritis, nausea, and vomiting.[17,18]
Aim and objectives
Keeping the benefit of epidural dexmedetomidine in consideration, we designed a prospective, randomized double blinded study to evaluate and compare its anesthetic effects and postoperative pain relief with epidurally administered fentanyl in patients undergoing lower limb surgeries.
METHODS
After obtaining the research ethics committee approval and the informed and written consent, 100 patients of both genders, aged 21–56 years, physical status American Society of Anaesthesiologist (ASA) I and II who underwent lower limb orthopedic surgery, were enrolled into the present study. Patients with diabetes mellitus, cardiac disease, hypertension, chronic obstructive respiratory disease, coagulation abnormalities, spinal deformities, and patients allergic to amide type of local anesthetics were excluded from the study. Patients were divided randomly into two groups: Group Ropivacaine+Dexmedetomidine (RD) and Group Ropivacaine+Fentanyl (RF), comprising of 50 patients each. All patients were premedicated with oral ranitidine 150 mg and alprazolam 0.25 mg a night before and 2 hour before on the morning of surgery. Patients were thoroughly counseled during the pre-operative evaluation and were properly explained about the nature of study before taking the written consent.
In the operation theatre, a good venous access was secured with 18G cannula and all the patients were prehydrated with 10 ml/kg of lactated Ringer's solution. All the baseline parameters were observed and recorded which consisted of electrocardiography (ECG), heart rate (HR), non-invasive blood pressure (NIBP), and pulse oximetry (SpO2).
Lumbar epidural anesthesia was induced using 18G Touhy needle with patients in the sitting position in L3-L4 interspace and location of epidural space was confirmed by loss of resistance technique. A test dose of 3 ml of 2% lignocaine with adrenaline was administered into epidural space and thereafter epidural catheter was secured 3–5 cm into the epidural space and patients were placed supine. The study solutions were prepared by an anesthesia technician who was given written instructions and was unaware of the study design. The following solutions were randomly administered: 15 ml of 0.75% ropivacaine associated to 1 μg/kg of dexmedetomidine in group RD (n=50) and 1 μg/kg of fentanyl in group RF (n=50) at the rate of 1 ml/second. The following parameters were observed immediately after the administration of epidural block.
Time to onset of analgesia at T10
Maximum sensory level achieved
Time to achieve the maximum sensory level
Time to complete motor blockade
Time to two segmental dermatomal regression
Regression to S2
First feeling of pain/rescue analgesia
Total dose consumption of local anaesthetic used over 24 hours.
Sedation was also assessed at intervals of 20 minutes intra-operatively and at intervals of 1 hour during post-op period using subjective sedation scale (Grade 0=awake, conscious, no sedation, to slightly restless; Grade 1=calm and compose; Grade 2=awake on verbal command; Grade 3=awake on gentle tactile stimulation; Grade 4=awake on vigorous shaking; Grade 5=unarousable). Motor blockade was assessed using modified Bromage scale (0=no block, 1=inability to raise extended leg, 2=inability to flex knee and 3=inability to flex ankle and foot) before surgery and at regular intervals of 1 hour post-operatively.
Power analysis was carried out before the initiation of study. An assumption of difference of 30 minutes in the post-operative analgesia between the RD and RF group with a power of 80% and α=0.05 was made which yielded a sample size of 43 patients per group.
Any untoward incident and side effects during the study period were carefully observed for and recorded and managed symptomatically. All the data are expressed as mean and standard deviation (SD) unless specified. At the end of study data was compiled systematically and was subjected to statistical analysis using statistical package for the social sciences (SPSS) version 15.0 for windows and employing analysis of variance (ANOVA) with post hoc significance, Chi-square test and Fisher's exact test for sedation and analgesia. Value of P<0.05 was considered significant and P<0.001 as highly significant.
RESULTS
A total of 100 patients who underwent lower limb surgery were enrolled for the study and were randomly divided into two groups. The demographic characteristics in both the groups exhibited marked similarities and did not show any statistical significant difference (P>0.05) [Table 1].
Table 1.
The demographic profile of the patients of both the groups
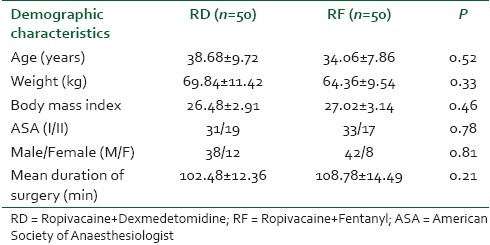
The onset of analgesia at T10 dermatomal level was significantly earlier in the RD group (7.12±2.44) as compared to the RF group (9.14±2.94). (P=0.016) The other early block characteristics also exhibited similar results as dexmedetomidine not only provided a higher dermatomal spread but also helped in achieving the maximum sensory anaesthetic level in a shorter period (13.38±4.48) as compared to Fentanyl (16.61±4.36). (P=0.021) Motor block was assessed using modified Bromage scale and complete motor block was achieved significantly earlier in the (18.16±4.52) patients who were administered dexmedetomidine as compared to RF group (22.98±4.78). (P=0.033) [Table 2].
Table 2.
The comparison of initial block characteristics in both the groups
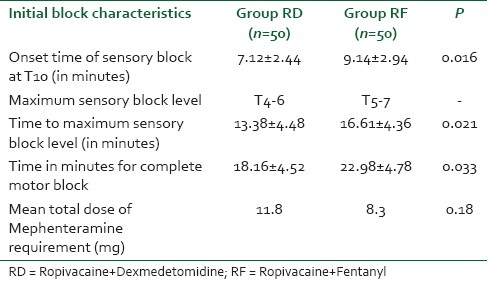
Dexmedetomidine has gained a lot of popularity as a sedative agent and similar findings were observed in our study as 38% and 42% of patients exhibited grade II and grade III sedation as compared to 16% and 2% of patients in the RF group, respectively. These sedation scores were highly significant on statistical comparison (P<0.001). Only 12% of the patients in the RD group had sedation scores of 1 as compared to 82% wide and awake patients in RF group which was a highly significant statistical entity (P<0.001) [Table 3].
Table 3.
The comparison of intra-operative sedation scores in patients of groups RD and RF
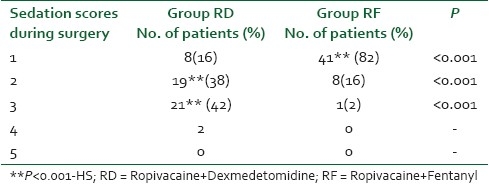
The finding of Table 4 reveals statistically significant values on comparison of post-operative block characteristics among the two groups. Though both the adjuvants provided a smooth and prolonged post-operative analgesia but the effects of dexmedetomidine were more significant on statistical comparison as compared to fentanyl. The evidence was very much visible in the prolonged time to two segmental dermatomal regression (140.32±10.21 in RD vs 110.84±9.48 in RF) (P=0.004) as well as earlier return of motor power to Bromage I in the RF group (178.52±23.29) as compared to RD group patients (259.62±21.38) (P=0.009). As a result, the time for rescue analgesia was comparatively shorter (242.16±23.86) in the patients who were administered fentanyl as compared to RD group who experienced prolonged pain free period (366.62±24.42) (P=0.012). The superior block characteristics by the addition of dexmedetomidine were clearly evident from the lesser dose consumption (76.82±14.28) of ropivacaine for postoperative analgesia for the next 24 hours (P=0.026).
Table 4.
The comparison of post-op block characteristics in both the groups
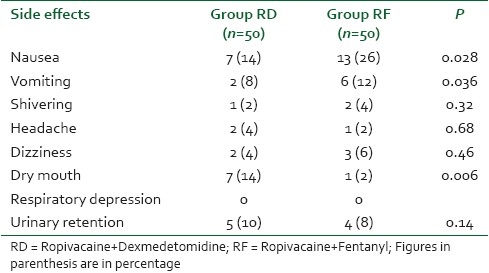
Table 5 shows the comparative incidence of various side effects in both the groups which were observed in the intra-op and post-op period. Nausea (26%) and vomiting (12%) were observed to a significant extent in the RF group with a comparative population of just 14 and 4%, respectively, in the RD group (P<0.05). The incidence of dry mouth was significantly higher in the RD (14%) group as compared to the RF group. (P=0.006) The incidence of other side effects like headache, shivering, dizziness, and urinary retention were comparable in both the groups and statistically non-significant (P>0.05). We did not observe respiratory depression in any of the patient from either group.
Table 5.
The comparison of side effects observed in both the groups during and after the operative period
DISCUSSION
Epidural analgesia offers superior pain relief and early mobilization especially when local anesthetic dose is combined with an adjuvant as compared to LA used alone.[2] Selection of exclusive epidural route during this study was done deliberately to avoid invasive dural penetration technique with spinal needle as well as to provide post-op pain relief. The synergism between epidural local anesthetics and opioids is well established but evidence regarding combination of LA with dexmedetomidine through epidural route is scarce in literature.[19,20] This is the pioneer study which has directly compared the effects of epidurally administered dexmedetomidine and fentanyl.
The demographic profile in the present study was comparable to similar other studies and did not show any significant difference on statistical comparison. The time to reach peak sensory level was significantly (P=0.021) shorter in group RD (13.38±4.48) as compared to group RF (16.61±4.36) as equally was the strikingly significant difference between the two groups regarding onset of sensory analgesia at T10 dermatomal level. Throughout the surgery, patients were calm and compose in both the groups but sedation scores were better in a highly significant manner in the RD group as 38% and 42% of patients had grade II and III sedation scores during the peri operative period as compared to 16% and 2% of patients in the RF group. The sedative properties of dexmedetomidine are far superior to fentanyl as no patient required any other sedative during the peri-operative period. None of the patients in either of the group required any additional epidural top-up dose during the surgical period. The analgesia was assessed using visual analogue scale (VAS) and patients in both the groups showed 0 scores during the entire surgical period. In our study, remarkable synergistic properties of LA and dexmedetomidine have come to the fore. Not only we were able to decrease the dose of local anesthetic in both the groups but also the duration of post-operative analgesia was significantly prolonged in patients in whom dexmedetomidine was administered as adjuvant with LA.
Though none of the patients in either of the groups experienced any respiratory difficulty warranting active intervention but patients in group RD exhibited a significantly a lower PaCO2 post operatively. The discrepancy can be explained on the basis that there have been no earlier studies which have established the dose equivalence of fentanyl and dexmedetomidine. Also, the dose of fentanyl used by us may be relatively lower than the equivalent dose of dexmedetomidine.
Bromage scale 3 was achieved in all the patients before the initiation of surgical procedure. The return of complete motor recovery was significantly earlier in the RD group as compared to RF group. Post-operatively, the number of analgesic top-up doses of ropivacaine in group RD was significantly lower than the requirement for ropivacaine in group F.
Hemodynamic stability was one of the most remarkable features observed with addition of dexmedetomidine and fentanyl to epidural ropivacaine. Decrease in heart rate is a known clinical effect of opioids but in the present study similar negative chronotropic effect was exhibited by dexmedetomidine approximately 30–35 minutes after the epidural injection of the drugs. [Figure 1] Thereafter, the heart rate remained stable in the range of 56–70/min in both the groups. Similarly, mean arterial pressure (MAP) decreased from the baseline in both the groups with a maximum decline of MAP at 30-50 minutes after the epidural injection but it never went below 65 mmHg [Figure 2]. Postoperatively, HR and MAP remained stable in both the groups. The decrease in HR caused by α-2 agonist can again be explained on the basis of their central action whereby they decrease sympathetic outflow and nor-epinephrine release.[14–16] The requirement of vasopressors for maintenance of stable hemodynamic parameters did not reveal any significant difference between both the groups on statistical comparison. The stable hemodynamics can possibly be explained on the basis of lower volume of local anesthetics used and a suitable selection of the dose of adjuvant.
Figure 1.

The comparison of heart rate (HR) in the group RD and RF covering the pre-, intra-, and post-operative period
Figure 2.

The comparison of mean arterial pressure (MAP) in the group RD and RF covering the pre-, intra-, and post-operative period
The side effect profile of both the groups exhibited a strikingly significant picture. Nausea and vomiting occurred in 26% and 14% of the patients in group RF as compared to 14% and 4% in group RD. This higher incidence of nausea and vomiting was observed despite a low dose of fentanyl used epidurally. Dry mouth is a known side effect of α-2 agonists and the incidence in the present study was found among 14% of the patients in group RD, which is quite similar to the observations of other studies administering dexmedetomidine. Although urinary retention is a known side effect of opioids, surprisingly we observed a higher incidence of urinary retention in group RD (10%) as compared to (8%) group RF patients. This discrepancy could not be explained and most probably the lower incidence of urinary retention in RF group can be attributable to a lower dose of fentanyl used in the present study.
Similarly, the absence of respiratory depression in the present study can be explained on the basis that fentanyl is less likely to induce respiratory depression as compared to morphine and we also used fentanyl in a lower dosage. As far as α-2 agonists are concerned, the respiratory depression is not a known feature of this group of drugs. The background of the present study mainly revolved around the potential side effects of epidural opioids and the available literature for intravenous dexmedetomidine has established a significant dose sparing action of the latter on opioid requirement after general anesthesia.[21,22]
Avoidance of respiratory depression in the patients who were administered dexmedetomidine was one of the most remarkable observations and the evidence is similar to the earlier studies where researchers have found complete absence of clinically detectable respiratory depression in the previous multiple human studies.[17,23,24] One big limitation of the present study involves the exact dose equivalence of dexmedetomidine and fentanyl when used in epidural anesthesia.
CONCLUSIONS
Dexmedetomidine seems to be a better alternative to fentanyl as an epidural adjuvant as it provides comparable stable hemodynamics, early onset and establishment of sensory anesthesia, prolonged post-op analgesia, lower consumption of post-op LA for epidural analgesia, and much better sedation levels.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schultz AM, Werba A, Ulbing S. Perioperative thoracic epidural analgesia for thoracotomy. Eur J Anaesthesiol. 1997;14:600–3. doi: 10.1046/j.1365-2346.1994.00183.x. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H. Acute pain control and accelerated postoperative surgical recovery. Surg Clin North Am. 1999;79:431–43. doi: 10.1016/s0039-6109(05)70390-x. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw BG, Liu SS, Thirlby RC. Standardized perioperative care protocols and reduced length of stay after colon surgery. J Am Coll Surg. 1998;186:501–6. doi: 10.1016/s1072-7515(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227–30. doi: 10.1046/j.1365-2168.1999.01023.x. [DOI] [PubMed] [Google Scholar]
- 5.Zaric D, Nydahl PA, Philipson L, Samuelsson L, Heierson A, Axelsson K. The effect of continuous lumbar epidural infusion of ropivacaine (0.1%, 0.2%, and 0.3%) bupivacaine on sensory and motor block in volunteers: A double-blind study. Reg Anesth. 1996;21:14–25. [PubMed] [Google Scholar]
- 6.McClellan KJ, Faulds D. Ropivacaine: An update of its use in regional anesthesia. Drugs. 2000;60:1065–93. doi: 10.2165/00003495-200060050-00007. [DOI] [PubMed] [Google Scholar]
- 7.Benzon HT, Wong HY, Belavic AM, Jr, Goodman I, Mitchell D, Lefheit T, et al. A randomized double-blind comparison of epidural fentanyl infusion versus patientcontrolled analgesia with morphine for postthoracotomy pain. Anesth Analg. 1993;76:316–22. [PubMed] [Google Scholar]
- 8.Salomaki TE, Laitinen JO, Nuutinen LS. A randomized doubleblind comparison of epidural versus intravenous fentanyl infusion for analgesia after thoracotomy. Anesthesiology. 1991;75:790–5. doi: 10.1097/00000542-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzini C, Moreira LB, Ferreira MB. Efficacy of ropivacaine compared with ropivacaine plus sufentanil for postoperative analgesia after major knee surgery. Anaesthesia. 2002;57:424–8. doi: 10.1046/j.0003-2409.2001.02393.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu SS, Allen HW, Olsson GL. Patient-controlled epidural analgesia with bupivacaine and fentanyl on hospital wards: Prospective experience with 1,030 surgical patients. Anesthesiology. 1998;88:688–95. doi: 10.1097/00000542-199803000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Mann C, Pouzeratte Y, Boccara G, Peccoux C, Vergne C, Brunat G, et al. Comparison of intravenous or epidural patient controlled analgesia in the elderly after major abdominal surgery. Anesthesiology. 2000;92:433–41. doi: 10.1097/00000542-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia: Their role in postoperative outcome. Anesthesiology. 1995;82:1474–506. doi: 10.1097/00000542-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–70. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 15.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine: A novel alpha2-adrenoceptor agonist–in healthy volunteers. Pain. 1991;46:281–5. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]
- 16.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 17.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloor BC, Abdul-Rasool I, Temp J, Jenkins S, Valcke C, Ward DS. The effects of medetomidine, an alpha2-adrenergic agonist, on ventilatory drive in the dog. Acta Vet Scand Suppl. 1989;85:65–70. [PubMed] [Google Scholar]
- 19.Soto RG, Fu ES. Acute pain management for patients undergoing thoracotomy. Ann Thorac Surg. 2003;75:1349–57. doi: 10.1016/s0003-4975(02)04647-7. [DOI] [PubMed] [Google Scholar]
- 20.Burmeister MA, Gottschalk A, Wilhelm S, Schroeder F, Becker C, Standl T. Ropivacaine 0.2% versus bupivacaine 0.125% plus sufentanil for continuous peridural analgesia following extended abdominal operations. Anasthesiol Intensivmed Notfallmed Schmerzther. 2001;36:219–23. doi: 10.1055/s-2001-12751. [DOI] [PubMed] [Google Scholar]
- 21.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 22.Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in postoperative patients needing sedation in the intensive care unit. Br J Anaesth. 2001;86:650–6. doi: 10.1093/bja/86.5.650. [DOI] [PubMed] [Google Scholar]
- 23.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 24.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]


