Figure 7.
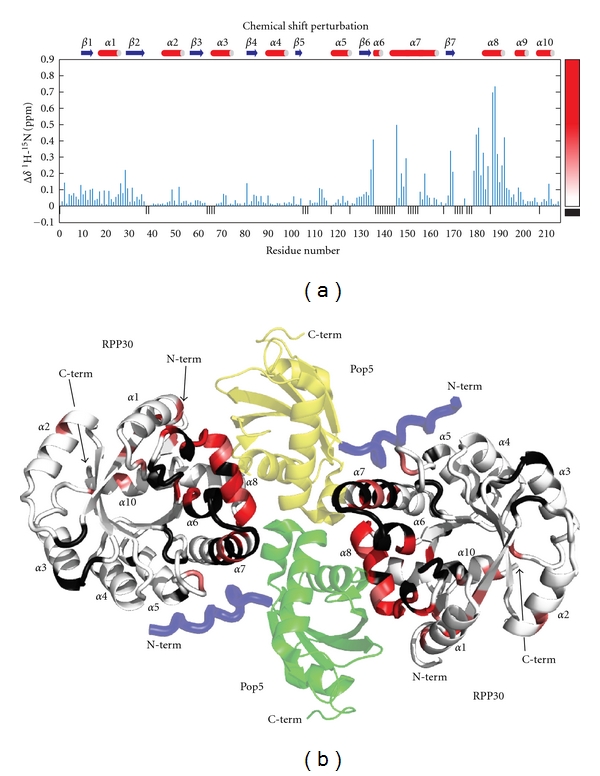
Chemical shift perturbations on RPP30 induced by Pop5 binding. (a) Secondary structure and per residue weighted average amide 1H and 15N chemical shift perturbations [20]. The color gradient used for mapping the data to the structure is shown on the right. Small black negative bars indicate residues for which the free and Pop5-bound RPP30 shifts could not be compared due to incomplete assignments or proline residues. (b) Cartoon diagram of a homology model of the Pfu Pop5-RPP30 complex based on the crystal structure of the complex from Pho [14]. Protomers of RPP30 are colored from white to red according to increasing shift perturbation. Black indicates no CSP data. The two protomers of Pop5 in the heterotetramer are green and yellow. An N-terminal segment observed in the Pho Pop5-RPP30 crystal structure, but not the Pfu Pop5 structure, is shown in blue.
