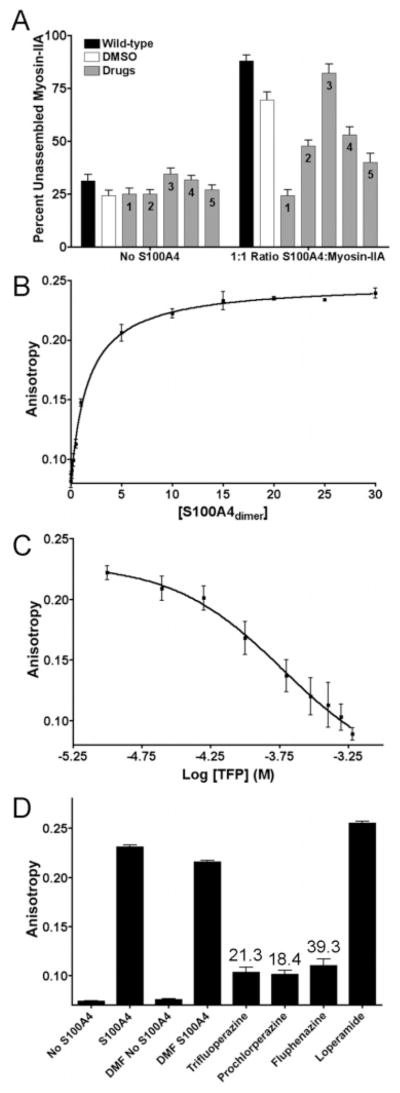
Figure 7.
Hit compounds disrupt the interaction of S100A4 and myosin-IIA. (A) A sedimentation assay was used to examine the ability of hit compounds to attenuate the destabilization of myosin-IIA filaments by S100A4. At a molar ratio of one wild-type S100A4 dimer per myosin-IIA rod, ~90% of the myosin-IIA rods are recovered in the supernatant. Values represent the mean and the standard error of the mean from at least four independent experiments. 1, trifluoperazine; 2, prochlorperazine; 3, loperamide; 4, bepridil, and 5, fluphenazine. (B) Fluorescence anisotropy measurements of S100A4 binding to FITC-MIIA1908–1923. Values represent the mean and the standard deviation from three independent experiments. A Kd of 1.7 ± 0.2 μM was determined from the fit to a single site saturation binding curve. (C) Titration experiment with trifluoperazine showing a loss of anisotropy as TFP competes off the bound FITC-MIIA1908–1923. The values represent the mean and the standard deviation from three independent experiments. The data were fit to a three parameter logistic equation to obtain the EC50. (D) Bar graph representation of anisotropy competition assays with lead compounds. Values represent the mean and the standard deviation for the endpoint of the titration (600 μM compound) from three independent experiments. The numbers above the bar indicate the Ki in units of μM.