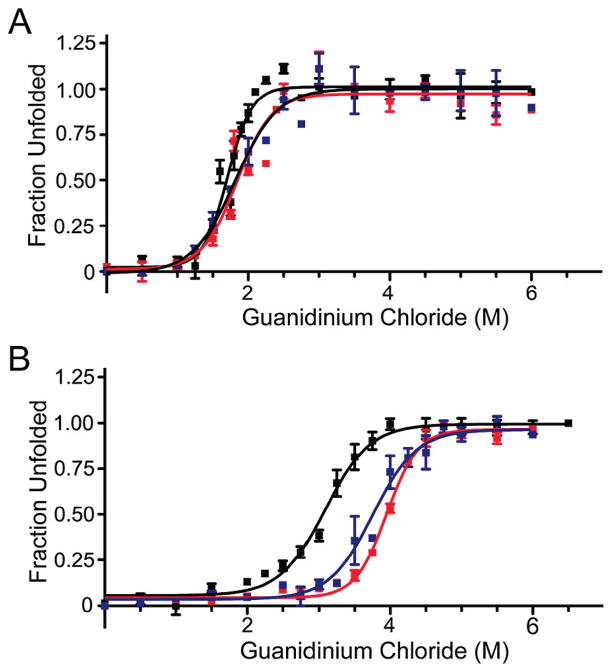
Figure 3.
Guanidine hydrochloride denaturation of sc-S100A4 proteins. (A) Unfolding of 5 μM wild-type S100A4 dimer or sc-S100A4 proteins in the presence of 2 mM EGTA. wt-S100A4, sc-S100A4–5, and sc-S100A4–10 exhibited similar midpoints of denaturation. (B) Unfolding in the presence of 2 mM CaCl2. The sc-S100A4 proteins were more resistant to denaturant-induced unfolding than wt-S100A4. wt-S100A4 (black), sc-S100A4–5 (red), and sc-S100A4–10 (blue). Values represent the mean ± standard error of the mean from three or four independent experiments.