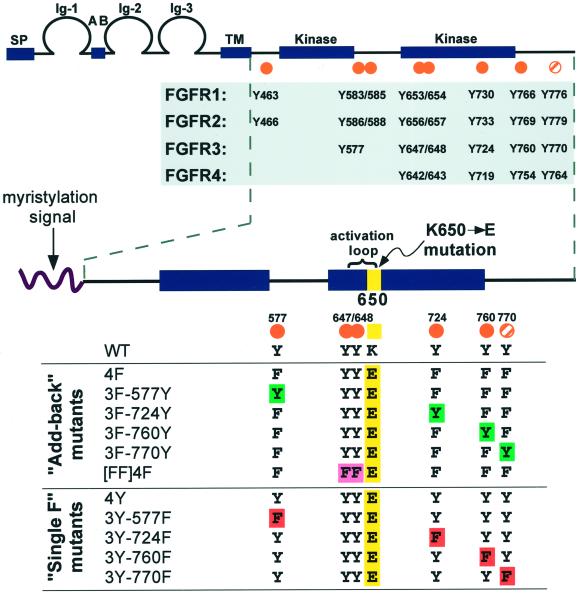
Figure 1.
FGFR conserved tyrosine residues and constructs designed for this study. (Top) General FGFR structure and conserved tyrosine residues. The seven autophosphorylation sites (Mohammadi et al., 1996a) mapped in FGFR1 are indicated by orange dots, and an eighth tyrosine (Y776) that is conserved in all four FGFRs is marked by an orange striped dot. (Bottom) Constructs generated for this study. A myristylation signal targets the intracellular domain of FGFR3 to the plasma membrane. All derivatives, with the exception of WT, contain the K650E point mutation (yellow). The [FF]4F construct contains mutations of the Y647 and Y648 activation loop residues to phenylalanine, as indicated by [FF] (pink). The 4F construct was used to derive the “Add-back” mutants, where each tyrosine was individually added back to the phenylalanine mutants (green). The 4Y derivative served as the parental constructs for the “Single F” derivatives, which have each nonactivation loop tyrosine individually removed by mutation to phenylalanine (red).