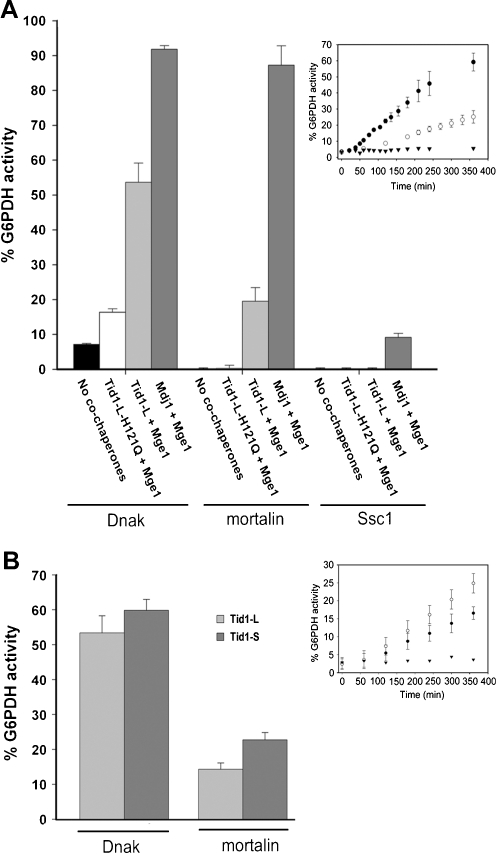
Fig. 3.
Yield of G6PDH refolding (after 6 h) obtained by DnaK, Ssc1, and mortalin. a Stable G6PDH aggregates were reactivated at 30°C in the presence of DnaK, Ssc1, or mortalin (16 μM), either alone or assisted by the indicated J-protein (2 μM) and Mge1 (1 μM). The presented values are averages of at least three different experiments with the background activity subtracted. The inset describes the time-dependent G6PDH activity in the presence of 16 μM DnaK (filled circles) or mortalin (empty circles) assisted by 2 μM Tid1-L and 1 μM Mge1. The spontaneous refolding of the aggregates in the presence of the reaction buffer is also shown (filled inverted triangles). b Same conditions as in (a), except that 1 μM of Tid1-L or Tid1-S was used. The inset describes the time-dependent G6PDH activity in the presence of 16 μM mortalin assisted by either 1 μM of Tid1-L (filled circles) or 1 μM of Tid1-S (empty circles) and 1 μM Mge1