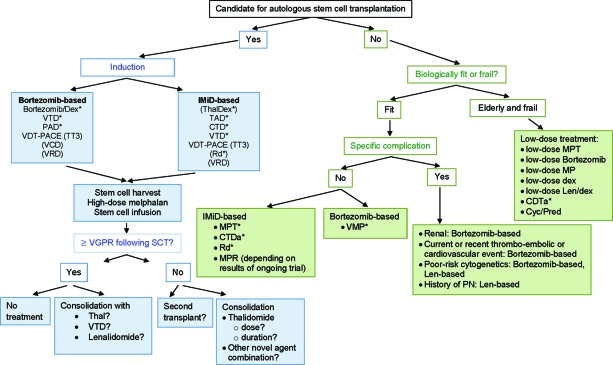
Figure 1.
MM treatment tree outside clinical trials: front line.
*Indicates data available from a phase III randomized trial.
Abbreviations: CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; Cyc, cyclophosphamide; Dex, dexamethasone; IMiD, immunomodulatory drug; Len, lenalidomide; MM, multiple myeloma; MP, melphalan plus prednisone; MPR, melphalan, prednisone, and lenalidomide; MPT, melphalan, prednisone, and thalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PN, peripheral neuropathy; Pred, prednisone; Rd, lenalidomide plus low-dose dexamethasone; SCT, stem cell transplant; TAD, thalidomide, doxorubicin, and dexamethasone; Thal, thalidomide; TT3, Total Therapy 3; VCD, bortezomib, cyclophosphamide, and dexamethasone; VDT-PACE, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VRD, bortezomib, lenalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.