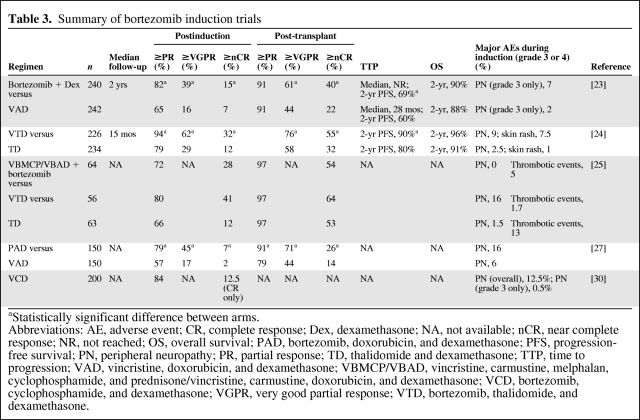
Table 3.
Summary of bortezomib induction trials
aStatistically significant difference between arms.
Abbreviations: AE, adverse event; CR, complete response; Dex, dexamethasone; NA, not available; nCR, near complete response; NR, not reached; OS, overall survival; PAD, bortezomib, doxorubicin, and dexamethasone; PFS, progression-free survival; PN, peripheral neuropathy; PR, partial response; TD, thalidomide and dexamethasone; TTP, time to progression; VAD, vincristine, doxorubicin, and dexamethasone; VBMCP/VBAD, vincristine, carmustine, melphalan, cyclophosphamide, and prednisone/vincristine, carmustine, doxorubicin, and dexamethasone; VCD, bortezomib, cyclophosphamide, and dexamethasone; VGPR, very good partial response; VTD, bortezomib, thalidomide, and dexamethasone.