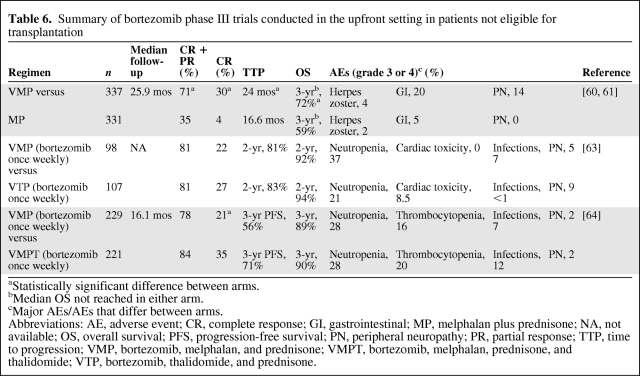
Table 6.
Summary of bortezomib phase III trials conducted in the upfront setting in patients not eligible for transplantation
aStatistically significant difference between arms.
bMedian OS not reached in either arm.
cMajor AEs/AEs that differ between arms.
Abbreviations: AE, adverse event; CR, complete response; GI, gastrointestinal; MP, melphalan plus prednisone; NA, not available; OS, overall survival; PFS, progression-free survival; PN, peripheral neuropathy; PR, partial response; TTP, time to progression; VMP, bortezomib, melphalan, and prednisone; VMPT, bortezomib, melphalan, prednisone, and thalidomide; VTP, bortezomib, thalidomide, and prednisone.