Abstract
A model is proposed for the processing of oligomeric viroid replication intermediates into monomeric, circular progeny viroids. The model identifies a thermodynamically extremely stable base-paired configuration that partially or completely dimeric, as well as higher, viroid oligomers can assume and postulates that this structure, which involves structural features common to all viroids (the central conserved region and secondary hairpin I), is essential for precise cleavage and ligation. The model explains why recombinant plasmids containing tandem repeats of two or more viroid sequence equivalents are highly infectious when inoculated into viroid-susceptible plants, why certain plasmids containing partially duplicated viroid-specific inserts are less infectious, and why plasmids containing monomeric inserts are noninfectious or at best marginally infectious. The model also accounts for the fact that vector-derived sequences on either or both sides of the viroid sequence(s) of a restriction fragment are precisely excised and are lacking in progeny viroids.
Full text
PDF

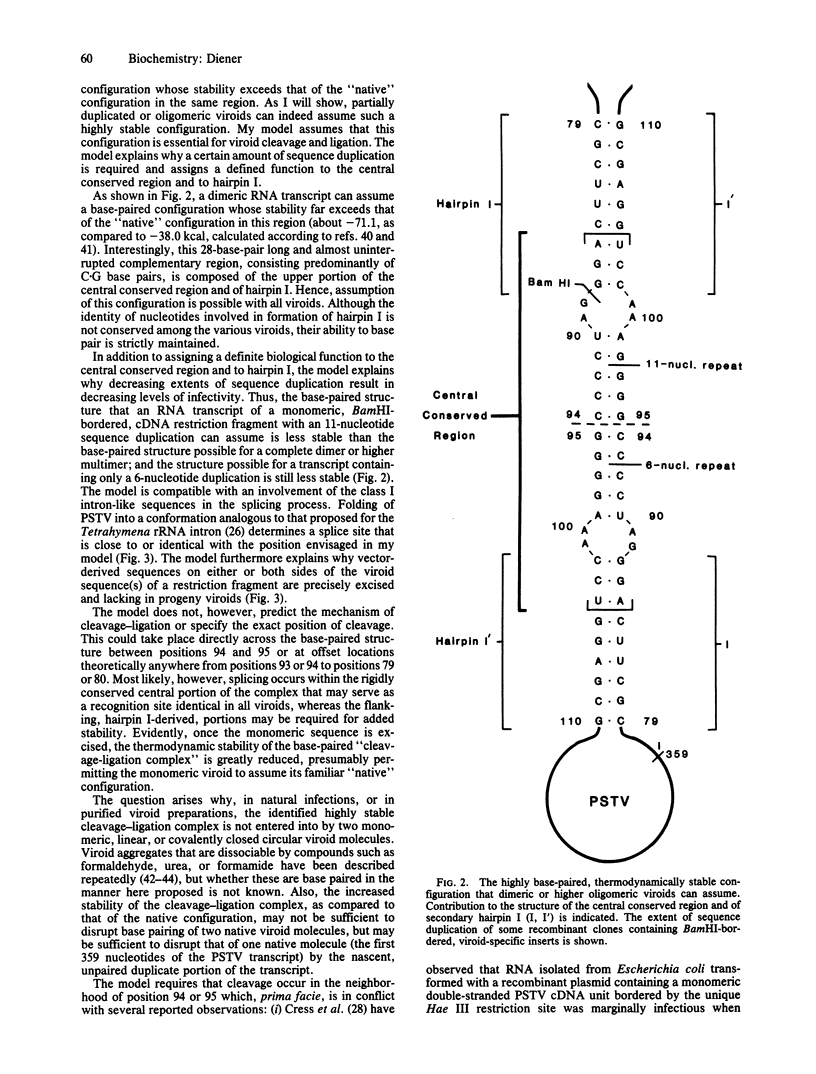

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress D. E., Kiefer M. C., Owens R. A. Construction of infectious potato spindle tuber viroid cDNA clones. Nucleic Acids Res. 1983 Oct 11;11(19):6821–6835. doi: 10.1093/nar/11.19.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Are viroids escaped introns? Proc Natl Acad Sci U S A. 1981 Aug;78(8):5014–5015. doi: 10.1073/pnas.78.8.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids. Adv Virus Res. 1983;28:241–283. doi: 10.1016/s0065-3527(08)60725-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids: structure and function. Science. 1979 Aug 31;205(4409):859–866. doi: 10.1126/science.472709. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Lossow C., Jank P., Raba M., Alberty H., Sänger H. L. Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature. 1978 May 18;273(5659):203–208. doi: 10.1038/273203a0. [DOI] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Cress D. E., Diener T. O. Nuclear DNA from uninfected or potato spindle tuber viroid-infected tomato plants contains no detectable sequences complementary to cloned double-stranded viroid cDNA. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6932–6935. doi: 10.1073/pnas.78.11.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Chrysanthemum stunt viroid: primary sequence and secondary structure. Nucleic Acids Res. 1981 Jun 25;9(12):2741–2752. doi: 10.1093/nar/9.12.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Sänger H. L., Riesner D. Fine structure melting of viroids as studied by kinetic methods. Nucleic Acids Res. 1979 Jul 11;6(9):3041–3059. doi: 10.1093/nar/6.9.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit length RNA in viroid replication. Mol Gen Genet. 1984;196(3):421–428. doi: 10.1007/BF00436189. [DOI] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Owens R. A., Diener T. O. Structural similarities between viroids and transposable genetic elements. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6234–6238. doi: 10.1073/pnas.80.20.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
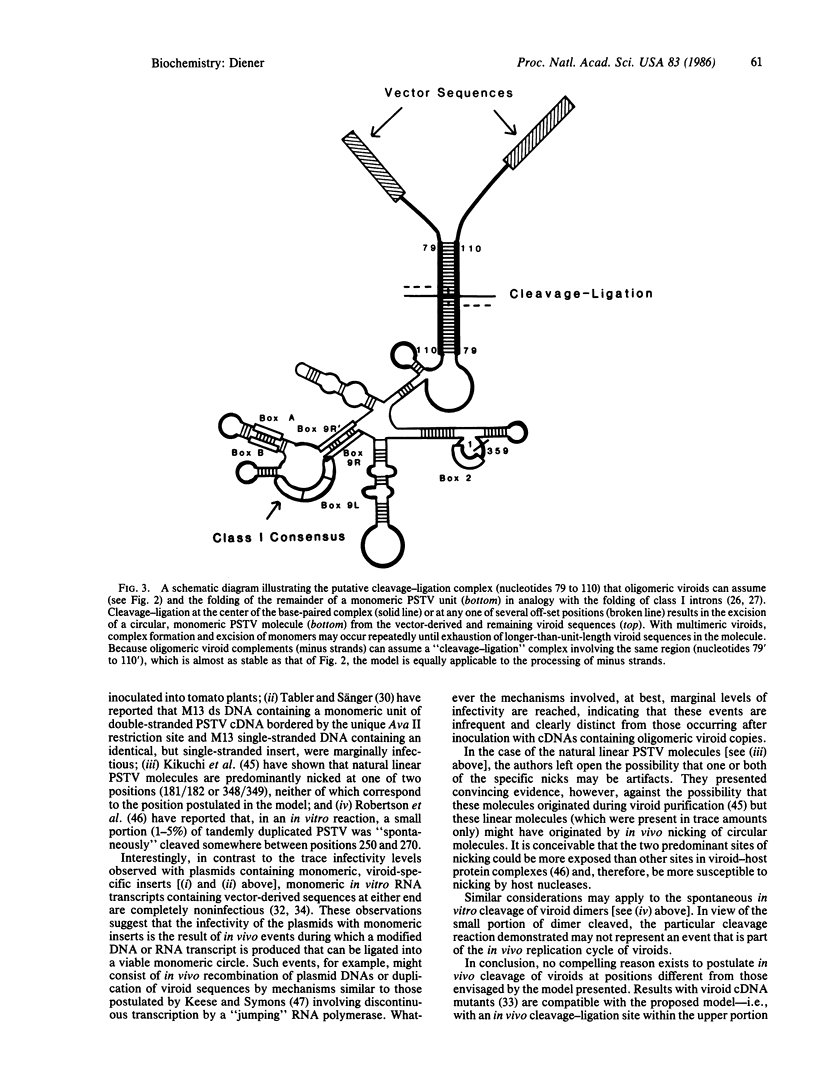
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Kruger K., Grabowski P. J., Zaug A. J., Sands J., Gottschling D. E., Cech T. R. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982 Nov;31(1):147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. Double-stranded cDNAs of hop stunt viroid are infectious. J Biochem. 1984 May;95(5):1521–1524. doi: 10.1093/oxfordjournals.jbchem.a134761. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci U S A. 1982 Jan;79(1):113–117. doi: 10.1073/pnas.79.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Erbe E., Hadidi A., Steere R. L., Diener T. O. Separation and infectivity of circular and linear forms of potato spindle tuber viroid. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3859–3863. doi: 10.1073/pnas.74.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Intervening sequences excised in vitro. Nature. 1978 Aug 10;274(5671):530–530. doi: 10.1038/274530a0. [DOI] [PubMed] [Google Scholar]
- Rohde W., Sänger H. L. Detection of complementary RNA intermediates of viroid replication by Northern blot hybridization. Biosci Rep. 1981 Apr;1(4):327–336. doi: 10.1007/BF01114872. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Michniewicz J. J., Narang S. A. Multiple forms of potato spindle-tuber metavirus ribonucleic acid. Can J Biochem. 1974 Sep;52(9):809–812. doi: 10.1139/o74-116. [DOI] [PubMed] [Google Scholar]
- Sogo J. M., Koller T., Diener T. O. Potato spindle tuber viroid. X. Visualization and size determination by electron microscopy. Virology. 1973 Sep;55(1):70–80. doi: 10.1016/s0042-6822(73)81009-8. [DOI] [PubMed] [Google Scholar]
- Steger G., Hofmann H., Förtsch J., Gross H. J., Randles J. W., Sänger H. L., Riesner D. Conformational transitions in viroids and virusoids: comparison of results from energy minimization algorithm and from experimental data. J Biomol Struct Dyn. 1984 Dec;2(3):543–571. doi: 10.1080/07391102.1984.10507591. [DOI] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Infectivity studies on different potato spindle tuber viroid (PSTV) RNAs synthesized in vitro with the SP6 transcription system. EMBO J. 1985 Sep;4(9):2191–2199. doi: 10.1002/j.1460-2075.1985.tb03914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Forster A. C., Symons R. H. Infectivity and in vitro mutagenesis of monomeric cDNA clones of citrus exocortis viroid indicates the site of processing of viroid precursors. Nucleic Acids Res. 1985 Aug 26;13(16):5843–5856. doi: 10.1093/nar/13.16.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J. E., Gould A. R., Bruening G. E., Symons R. H. Citrus exocortis viroid: nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982 Jan 25;137(2):288–292. doi: 10.1016/0014-5793(82)80369-4. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Symons R. H. Eleven new sequence variants of citrus exocortis viroid and the correlation of sequence with pathogenicity. Nucleic Acids Res. 1985 Apr 25;13(8):2907–2920. doi: 10.1093/nar/13.8.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P., Gilz R., Schumacher J., Riesner D. Complexes of viroids with histones and other proteins. Nucleic Acids Res. 1985 Jan 25;13(2):355–367. doi: 10.1093/nar/13.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G., Tabak H. F. Self-splicing of yeast mitochondrial ribosomal and messenger RNA precursors. Cell. 1985 Apr;40(4):759–766. doi: 10.1016/0092-8674(85)90335-6. [DOI] [PubMed] [Google Scholar]



