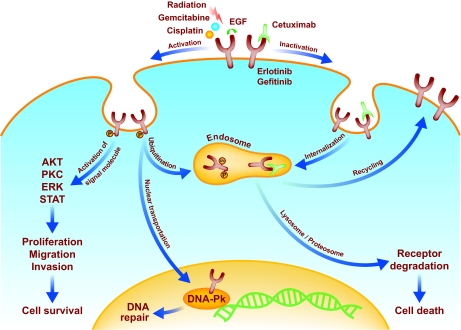
Figure 1.
The effects of radiation, chemotherapy, and targeted agents on EGFR signaling. After stimulation by irradiation or certain chemotherapeutic agents, epidermal growth factor receptor (EGFR) can activate downstream signaling pathways that can promote cell survival or cell death. In addition to stimulating the pathways activated by epidermal growth factor (EGF), radiation can trigger the translocation of phosphorylated EGFR (pEGFR) into the nucleus. This process coincides with the transport of Ku70/80 and protein phosphatase 1 into the nucleus (not shown), which results in increases in DNA-dependent protein kinase (DNAPK) levels, the repair of DNA-strand breaks, and cell survival. Cetuximab blocks nuclear transport of pEGFR; it binds to EGFR and causes endosome internalization, ultimately causing receptor degradation and cell death. Gefitinib and erlotinib bind to the intracellular ATP binding site of EGFR, thereby inhibiting unregulated EGFR signaling. Gemcitabine causes the phosphorylation of EGFR. In this case, EGFR phosphorylation initially activates Akt promoting cell survival, but subsequently promotes the ubiquitination (Ub) of the receptor, which leads to its degradation along a proteosome or lysosome pathway. pEGFR degradation results in the downregulation of the survival signal pAkt, leading to apoptosis. Blocking EGFR degradation at various steps of this pathway reduces gemcitabine-mediated cytotoxicity. Whether an EGFR-activating insult leads to cell survival or cell death might ultimately be determined by the severity and duration of the stress.