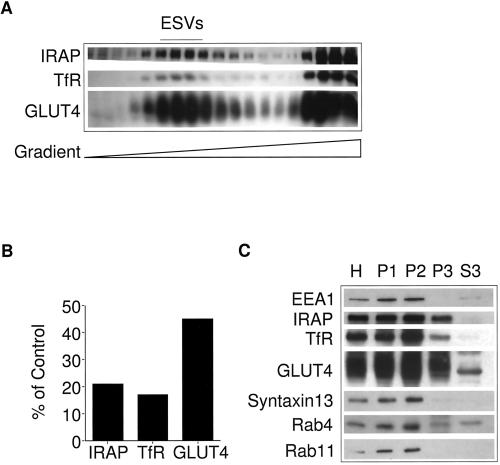
Figure 1.
Identification of ESVs in CHO cells stably transfected with myc epitope-tagged GLUT4 at steady state. (A) Western blot analysis of fractions generated by velocity sedimentation. Homogenates from CHO/GLUT4myc cells were fractionated by velocity sedimentation on 5–25% glycerol gradients over a 50% sucrose pad and centrifuged at 60,000 × g for 75 min. Note that GLUT4, IRAP, and TfR colocalize with slowly sedimenting small vesicles (fractions 4–12) and larger, rapidly sedimenting membranes at the bottom of the gradient (fractions 15–19). (B) Densitometry analysis of A. For each marker, the total signal was calculated by summing the signal from each fraction. (C) Western blot analysis of fractions generated by differential centrifugation. Each fraction (20 μg) was analyzed: H, homogenate; P1, a pellet centrifuged at 500 × g for 5 min (enriched in plasma membranes); P2, a pellet from the supernatant, obtained after a 500 × g spin, centrifuged at 27,000 × g for 45 min (enriched in endosomal membranes); P3, a pellet from the supernatant left behind by the P2 spin centrifuged at 100,000 × g for 1 h (enriched in small vesicles); S3, the supernatant left behind after the P3 spin. Note the inclusion of IRAP, GLUT4, TfR, cellubrevin, and Rab4 but the exclusion of syntaxin13, EEA1, and Rab11 from the small vesicular fractions. A sharp band of ∼45 kDa is observed in the S3 fraction. This band, lacking the pattern of the normally glycosylated GLUT4, appears to be a cross-reacting soluble protein and not GLUT4.