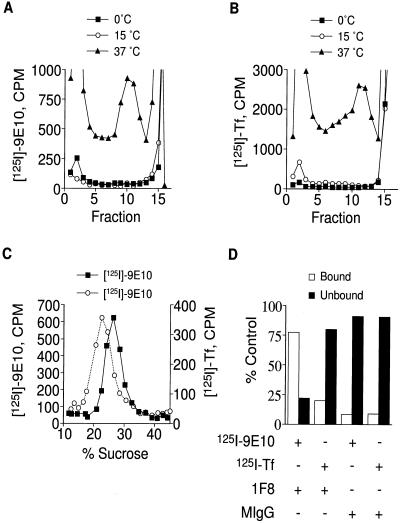
Figure 2.
Labeling of ESVs by internalization of radiolabeled ligands. (A and B) Velocity sedimentation of homogenates. CHO/GLUT4myc cells were incubated with 5 μg/ml 125I-9E10 (A) or 125I-Tf (B) for 30 min at 37°C, 80 min at 15°C, or 2 h at 0°C and fractionated as described for Figure 1. Note targeting of the radiolabeled ligands to similar sized ESVs as those seen in Figure 1. (C) Separation of ESVs by equilibrium density centrifugation. Peak fractions from the glycerol gradients containing labeled ESVs were layered onto 10–45% sucrose gradients and centrifuged at 183,000 × g for 18 h. Note the different buoyant densities for ESVs containing transferrin (∼22% sucrose) compared with ESVs containing 9E10 (∼27% sucrose). (D) Immunoisolation of labeled ESVs. Radiolabeled homogenates were centrifuged at 27,000 × g for 30 min. The supernatant, enriched in ESVs, was incubated with anti-GLUT4 tail antibody (1F8) or mouse IgG overnight at 4°C and then centrifuged on glycerol gradients as above. Peak fractions containing the ESVs were incubated with protein G-Sepharose beads overnight at 4°C. The amount of each ligand bound to the beads was compared with the amount of ligand left behind in the unbound supernatant. Note the targeting of each ligand to discrete types of small vesicles. + denotes presence in assay; − denotes absence.