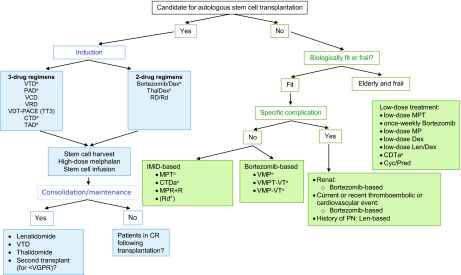
Figure 1.
Multiple myeloma treatment tree outside clinical trials: frontline.
aIndicates data available from phase III randomized trial.
Lenalidomide is currently not EMA approved for the treatment of newly diagnosed multiple myeloma or as consolidation or maintenance treatment. Bortezomib is currently not EMA approved for the treatment of transplant-eligible patients or as consolidation or maintenance treatment. Thalidomide is currently not EMA approved for the treatment of transplant eligible patients or as consolidation or maintenance treatment.
Abbreviations: CR, complete response; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; MP, melphalan and prednisone; MPR + R, melphalan, prednisone, and lenalidomide plus lenalidomide maintenance; MPT, melphalan, prednisone, and thalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PN, peripheral neuropathy; Pred, prednisone; Rd, lenalidomide plus low-dose dexamethasone; RD, lenalidomide and dexamethasone; TAD, thalidomide, doxorubicin, and dexamethasone; Thal, thalidomide; TT3, Total Therapy 3; VCD, bortezomib, cyclophosphamide, and dexamethasone; VDT-PACE, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VMPT-VT, bortezomib, melphalan, prednisone,and thalidomide followed by maintenance with bortezomib and thalidomide; VRD, bortezomib, lenalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.