A series of patients with locally advanced cervical cancer, with no positive para-aortic nodes on positron emission tomography–computed tomography who had undergone a primary laparoscopic para-aortic lymphadenectomy was retrospectively reviewed. Morbidity was limited and the completion of treatment was not delayed when complications occurred.
Keywords: Cervical cancer, Para-aortic lymphadenectomy, Laparoscopy, Staging, Morbidity, Lymphocyst
Abstract
Background.
Laparoscopic para-aortic lymphadenectomy (PAL) is being used increasingly to stage patients with locally advanced cervical cancer (LACC) and to define radiation field limits before chemoradiation therapy (CRT). This study aimed to define clinical implications, review complications, and determine whether surgical complications delayed the start of CRT.
Methods.
We retrospectively reviewed a continuous series of patients with LACC, with no positive para-aortic (PA) nodes on positron emission tomography–computed tomography (PET–CT) and who had undergone a primary laparoscopic PAL.
Results.
From November 2007 to June 2010, 98 patients with LACC underwent pretherapeutic PAL. Two patients did not undergo PAL: extensive carcinomatosis was discovered in one case and a technical problem arose in the other. No perioperative complications occurred. Seven patients had a lymphocyst requiring an imaging-guided (or laparoscopic) puncture. Eight patients (8.4%, which corresponds to the false-negative PET–CT rate) had metastatic disease within PA lymph nodes. In cases of suspicious pelvic nodes on PET–CT, the risk for PA nodal disease was greater (24.0% versus 2.9%). When patients with and without surgical morbidity were compared, the median delay to the start of treatment was not significantly different (15 days; range, 3–49 days versus 18 days; range, 3–42 days).
Conclusions.
The morbidity of laparoscopic PAL was limited and the completion of treatment was not delayed when complications occurred. Nevertheless, if PET–CT of the pelvic area is negative, the interest in staging PAL could be discussed because the risk for PA nodal disease is very low.
Introduction
Chemoradiation therapy (CRT), a combination of external irradiation and brachytherapy with concurrent chemotherapy, is considered the standard treatment for bulky cervical cancer (stage ≥IB2 according to the International Federation of Gynecology and Obstetrics [FIGO] classification) by many North American and western European teams [1]. The incidence of nodal metastasis is correlated with tumor volume and clinical stage [2]. When para-aortic (PA) nodes are known to be metastatic, the radiation field is extended from the pelvis to include the PA area [3]. This extended-field radiation therapy cannot be systematically delivered to all patients with locally advanced cervical cancer (LACC) because it generates significant morbidity, especially in patients previously submitted to a laparotomy [4]. Only 15%–30% of patients with LACC have PA metastasis [2, 5], consequently, the risk for overtreating patients with clinically negative PA lymph nodes is believed to outweigh the benefits of extended-field radiation in the minority of patients with undiagnosed PA lymph node metastasis. The sensitivity of computed tomography (CT) scan or magnetic resonance imaging (MRI) in detecting PA metastasis <1 cm is poor [6]. Currently, 18F-fluorodeoxyglucose positron emission tomography CT (PET–CT) is the best imaging method for detecting small-sized PA lymph node involvement [7, 8]. However, the rate of false negatives on PET–CT assessment of PA metastasis in LACC is 8%–13% [9, 10]. Indeed, current imaging modalities are not efficient enough to detect PA lymph node metastases, which remain undertreated.
The concept of surgical staging has gained momentum with the development of laparoscopy, which reduces surgical complications. This is why laparoscopic para-aortic lymphadenectomy (PAL) is being used increasingly to stage patients with LACC and to define radiation fields before CRT. The morbidity of this staging procedure must be low and CRT should not be delayed by surgery-related complications so that the benefits of staging are not offset by delaying the standard treatment. The aim of this study was to describe complications arising from surgical staging, discover how they were treated in our institution, and examine whether surgical complications delayed the beginning of CRT. In addition, we studied the sensitivity of PET–CT in detecting PA nodal disease.
Patients and Methods
Since 2007, in our institute, patients with LACC with no evidence of extrapelvic disease on preoperative imaging (MRI or CT scan) and with no obvious disease in the PA area on PET–CT who are <70 years old have undergone a pretherapeutic laparoscopic staging procedure to tailor their radiation therapy fields. This is a continuous series and no patients were excluded. LACC was defined according to the 2009 FIGO classification as clinical stage IB2–IVA [11]. The surgical staging procedure began with a transumbilical diagnostic laparoscopy to assess the peritoneal cavity. In the absence of i.p. disease, an extraperitoneal PA lymphadenectomy was performed via the left-sided extraperitoneal approach, as described in detail by Querleu et al. [5] and Dargent et al. [12]. At the beginning of our experience, some transperitoneal dissections were performed and then we exclusively performed extraperitoneal dissections, except if there was opening of the peritoneum and if simple sutures had failed to control peritoneal leakage.
The lymphadenectomy specifically targeted the left-sided supra- and inframesenteric PA space, which harbors most PA metastases from LACC [2]. If suspicious nodes were found during the procedure, they were sent for frozen section analysis, and if they were metastatic, the procedure was stopped and the radiation fields were extended to the PA region. Although pelvic nodal dissection is not part of the standard procedure, because these nodes are included in the radiation field, the removal of isolated enlarged pelvic nodes (>2 cm) was considered.
“Preventive marsupialization,” as described by Leblanc et al. [6] (transperitoneal opening of the left paracolic gutter to allow abdominal reabsorption of the retroperitoneal lymph) was performed after our early experience to try to avoid the development of postoperative symptomatic retroperitoneal lymphocysts.
Lymph nodes were formalin fixed and embedded in paraffin blocks after macroscopic dissection by a pathologist. Sections measuring 4 μm were cut and stained with hematoxylin and eosin. Each lymph node was examined microscopically to determine whether it harbored a metastasis and measure the size of the metastatic lesion. Serial sectioning and immunohistochemical analysis were performed when a micrometastasis was suspected.
Further management of the primary cervical cancer was individually tailored according to the results of the pretherapeutic staging procedure. Patients with negative PA lymph nodes received external-beam radiotherapy to the pelvis with conformal techniques up to a total dose of 45 Gy (five fractions of 1.8 Gy per week) with concurrent platinum-based chemotherapy (cisplatin, 40 mg/m2 per week). External-beam radiation therapy was followed by intracavitary, with or without interstitial, pulsed dose rate brachytherapy up to a total dose of at least 15 Gy to the intermediate-risk clinical target volume, as defined by the Groupe Européen de Curiethérapie and the European Society for Therapeutic Radiology and Oncology recommendations [13]. Limited boosts were individually delivered to clinically involved pelvic nodes diagnosed on initial imaging up to a total dose of 60–65 Gy, taking the contribution of brachytherapy into account. For patients with metastatic PA lymph nodes, radiation fields were extended to include the PA area up to a total dose of 45 Gy, with the same therapeutic schedule in the case of brachytherapy. Six to eight weeks after the completion of radiation therapy, patients underwent a clinical and MRI evaluation in search of residual disease. Follow-up was initiated in the absence of residual disease. Completion surgery was discussed when residual disease was suspected. Patients with carcinomatosis received platinum-based chemotherapy along with palliative radiotherapy. Patient follow-up consisted of serial clinical examinations by an oncologist every 4 months during the first year and every 6 months thereafter.
We reviewed all surgical complications and classified morbidity according to the Clavien and Dindo classification [14] from grade 1 (no specific treatment) to grade 5 (deceased).
We collected data on the time between PET–CT and surgery, between surgery and the start of CRT, and between surgery and the end of standard treatment.
Descriptive statistics were used to summarize the clinicopathologic characteristics of the study sample. A χ2 analysis and Student's t-test were used to compare nominal variables.
Results
From November 2007 to June 2010, 98 consecutive patients with stage IB2–IVA cervical cancer underwent a pretherapeutic laparoscopic staging procedure. Two patients did not undergo PAL because extensive carcinomatosis was discovered during the initial transperitoneal exploration in one case and a technical problem had arisen in the other (poor hemodynamic tolerance of pneumoperitoneum for an obese and severely asthmatic patient). The median age was 45 years (range, 21–65 years) and the median body mass index (BMI) was 23.0 kg/m2 (range, 14.4–42.7 kg/m2) for the 96 patients who completed the staging procedure. No patient was excluded because of obesity.
According to the 2009 FIGO classification [11], clinical stages were IB1 (n = 1) (this patient had positive pelvic nodes on PET–CT; she had undergone staging with PAL and a pelvic lymphadenectomy), IB2 (n = 35), IIB (n = 51), IIA2 (n = 2), IIIA (n = 1), IIIB (n = 4), and IVA (n = 2). The histologic types were squamous carcinoma (n = 85), adenocarcinoma (n = 7), clear cell adenocarcinoma (n = 2), glassy cell carcinoma (n = 1), and neuroendocrine small cell carcinoma (n = 1).
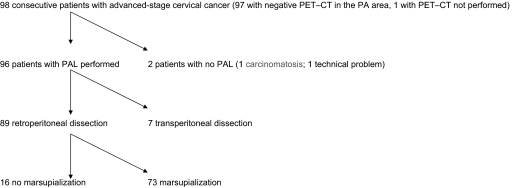
The flow chart of the study population with the imaging and surgical techniques used is detailed in Figure 1. Ninety-five of the 96 patients in the study underwent a PET–CT before surgical staging and there were no signs of metastatic PA nodes. Patients exhibiting positive nodes did not undergo surgical staging and the radiation field was extended to the PA area (clear PET–CT PA uptake with a significant standardized uptake value was considered sufficient to extend the field without a systematic biopsy by interventional radiology). Imaging was scheduled for the patient with no PET–CT, but she did not undergo the exam. The median interval between PET–CT and surgical staging was 13 days (range, 1–49 days).
Figure 1.
Flow chart of study population.
Abbreviations: PA, para-aortic; PAL, para-aortic lymphadenectomy; PET–CT, positron emission tomography–computed tomography.
Eighty-nine PALs were performed via retroperitoneal dissection and seven were performed exclusively via transperitoneal dissection (this approach was deliberately chosen in six patients but it was used because of failure to control a peritoneal leak with sutures in the seventh patient). The first 16 patients who had PAL via retroperitoneal dissection did not undergo “preventive marsupialization,” but the procedure was included in the following 73 patients.
The median operative time was 185 minutes (range, 105–480 minutes). The median and mean numbers of lymph nodes removed were 13 (range, 4–39) and 14.7, respectively. No perioperative major complications (and no laparotomic conversion) occurred. In particular, no intraoperative bowel injury, vessel injury, postoperative ileus, or bowel herniation occurred. No patient required blood transfusion.
The median postoperative hospital stay was 3 days (range, 2–12 days). Seven patients had grade 1–2 morbidity (postoperative pain, bleeding, and infection). Seven patients had grade 3 morbidity resulting from the procedure (10.7%) and one patient had grade 4 morbidity.
All cases of grade 3 morbidity were symptomatic lymphocysts requiring an imaging-guided puncture (they had all undergone a retroperitoneal PAL), and three of them had undergone a further surgical procedure via laparoscopy (laparoscopic opening of the peritoneum for a recurrent lymphocyst). Two of the three patients requiring further surgery had not undergone preventive marsupialization during the initial laparoscopy.
Regarding the case of grade 4 morbidity, this patient experienced acute renal failure (creatinemia at 400 μM/l and oligoanuria over 72 hours) and was transferred to the intensive care unit for 1 week. Regression was spontaneous with complete recovery of kidney function.
Among the seven patients who required a puncture, two had not undergone preventive marsupialization (those two patients also required laparoscopic opening of the peritoneum) and five had undergone marsupialization. One patient had metastatic positive nodes. Age, BMI, no preventive marsupialization, the number of nodes removed, and PA metastasis were not identified as risk factors for symptomatic lymphocyst formation.
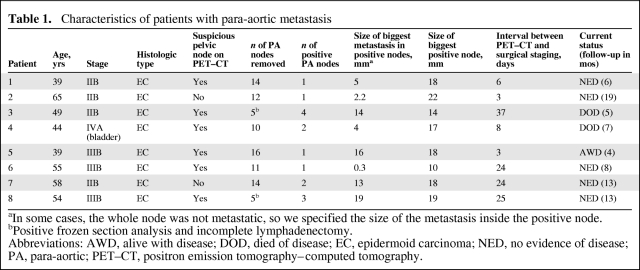
The definitive pathological analysis revealed that eight patients had metastatic disease within PA lymph nodes. The false-negative rate of PET–CT in this study was 8.4% (eight of 95 patients with PET–CT before surgery). Patient characteristics and the size and number of metastases in these eight patients are detailed in Table 1. The median interval between PET–CT and surgical staging for these eight patients was 16 days (range, 3–37 days). A frozen section analysis was performed for two patients during the procedure because of macroscopically suspicious PA nodes. Because the frozen section analysis was positive, the procedure was interrupted without completing the lymphadenectomy (these were the two patients in whom only five PA nodes were removed).
Table 1.
Characteristics of patients with para-aortic metastasis
aIn some cases, the whole node was not metastatic, so we specified the size of the metastasis inside the positive node.
bPositive frozen section analysis and incomplete lymphadenectomy.
Abbreviations: AWD, alive with disease; DOD, died of disease; EC, epidermoid carcinoma; NED, no evidence of disease; PA, para-aortic; PET–CT, positron emission tomography–computed tomography.
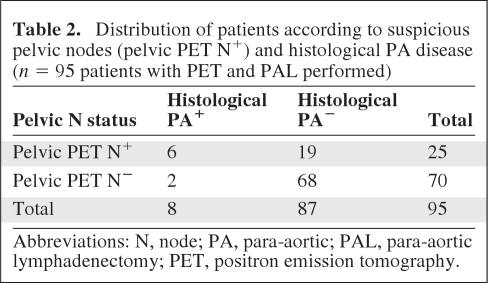
Among the 19 patients with suspicious pelvic nodes on PET–CT, only one (the one with stage IB1 disease) underwent a pelvic lymphadenectomy because the size of the pelvic node was >2 cm, which is supposed to be the limit for sterilization by radiotherapy (94% control rate achieved with 6,000 rads and cisplatin [15]). The pelvic nodal lesion removed was metastatic and it measured 3 cm (this node was removed via laparoscopy with no rupture). The PAL was negative in this patient.
When PET–CT depicted suspicious pelvic nodes, the risk for PA disease was greater (24.0% versus 2.9%; p = 0.004) (odds ratio, 10.7; confidence interval, 2.3–50.1) (Table 2).
Table 2.
Distribution of patients according to suspicious pelvic nodes (pelvic PET N+) and histological PA disease (n = 95 patients with PET and PAL performed)
Abbreviations: N, node; PA, para-aortic; PAL, para-aortic lymphadenectomy; PET, positron emission tomography.
The median intervals between surgery and the start of CRT and surgery and the end of standard treatment were 18 days (range, 3–49 days) and 71 days (range, 49–133 days), respectively. When patients with and without surgical morbidity were compared, the median intervals to the start of CRT and to the end of treatment were not significantly different—15 days (range, 3–49 days) versus 18 days (range, 3–42 days) and 66 days (range, 50–123 days) versus 72 days (49–133 days). The patient with a 49-day interval to the start of treatment was the one with acute kidney failure (chemotherapy was carboplatin instead of cisplatin). Except for this patient, CRT was never delayed because of surgical morbidity.
RT was extended to the PA area in all patients with positive PA nodes. The oncologic outcome of these patients is detailed in Table 1.
Discussion
The interest and impact of laparoscopic PA staging lymphadenectomy in the management of LACC continue to fuel debate. The rate of PA metastasis in LACC is 15%–20% [2, 5], and in the event of PA spread, the prognosis is drastically worse, with an overall survival rate of only 17% in the series reported by Delpech et al. [16] and Leblanc et al. [6]. Conventional imaging is able to detect <30% of PA spread [6]. An interesting randomized trial, performed by a team well versed in the management of cervical cancer, was published nearly 10 years ago. It compared surgical staging (a laparotomic or laparoscopic approach) with conventional imaging (before the era of PET–CT imaging) and demonstrated that surgical staging had a deleterious effect on survival [17]. However, the surgical morbidity rate in that trial was very high (>40%) and only 30 patients were included per arm [17]. Despite no difference in patient characteristics between the two arms, there was a tendency for patients with the worst prognosis (size of tumor, incidence of positive pelvic nodes, number of patients treated with concomitant chemotherapy) to be in the arm treated with surgical staging [17]. More recently, a paper by Gold et al. [18], concerning the impact of surgical staging on patients included in three randomized Gynecologic Oncology Group (GOG) trials, suggested that PA surgical staging afforded a benefit in terms of survival. Finally, in a study by Leblanc et al. [6], the survival duration of patients with involved PA nodes measuring <5 mm and diagnosed after laparoscopic retroperitoneal staging surgery (and treated with extended-field CRT) was similar to that of patients without PA nodal spread. In other words, there is probably an impact on survival of PA staging surgery (followed by extended-field CRT if PA nodes are positive), and particularly in the subgroup of patients with nodal involvement measuring <5 mm.
Is the PA nodal involvement detected on surgical staging actually depicted by PET–CT? This procedure is the most relevant imaging modality for the evaluation of distant spread from LACC [7, 8] and its use is currently recommended by many teams for the initial evaluation of all LACC cases. However, recent studies correlating the results of PET–CT and pathological examination of nodal involvement have reported a false-negative rate of ∼8%–12% [9, 10, 19, 20]. In particular, Ramirez et al. [10], in their study on 60 patients, observed that three of the 26 patients with negative pelvic and PA nodes on PET–CT had histopathologically positive PA nodes (12%). Of the 27 patients with positive pelvic but negative PA nodes on PET–CT, six (22%) had histopathologically positive PA nodes. Eleven (18.3%) patients had treatment modification based on surgical findings in that study [10].
In these different studies, nearly 40% of patients with false-negative PET–CT results had nodal metastasis <5 mm [10, 21, 22], and those patients were considered the best candidates for definitive cure of their metastatic disease by extended CRT fields. In one of the most recent collaborative studies, the sensitivity, specificity, positive predictive value, and negative predictive value of PET–CT were 33.3%, 94.2%, 53.8%, and 87.5%, respectively, for the detection of microscopic lymph node metastases [20].
The only way to definitely confirm the therapeutic impact of PA surgical staging (in patients with a negative PET–CT result in the PA area) is to conduct a randomized trial. However, the question is how many patients would have to be included? According to the results of the GOG 125 trial, which analyzed the survival of patients with PA spread treated with extended-field CRT, we can extrapolate that 40%–50% of patients with stage IB2 or II disease would be alive at 5 years [3]. The hypothetical gain in overall survival as a result of PA surgical staging would therefore concern 4%–5% of patients (i.e., 40%–50% of the 8%–12% of patients with false-negative PET–CT results). Ideally, a randomized trial should be performed to confirm such a therapeutic impact, but to test the benefit in terms of survival for PAL (with an α risk of 5% and a β risk of 10%), 4,600 patients would be required to demonstrate an overall survival benefit of 4% at 5 years after surgical staging (2,900 patients would be required for a 5% benefit). Clearly, this is unrealistic given the incidence of cervical cancer in developed countries.
In this context, surgical staging could nonetheless be acceptable only if “severe” morbidity (defined as morbidity that could delay the start of CRT) was extremely limited (i.e., <4%–5%, which is the potential impact on overall survival). A meta-analysis of 13 trials comparing CRT with the same radiotherapy alone confirmed that chemotherapy in the treatment of LACC patients leads to longer disease-free and overall survival times and lower rates of local and distant recurrence and progression [1]. CRT is therefore considered the standard treatment for bulky cervical cancer (stage ≥IB2 according to the FIGO classification) by many North American and western European teams. In our study, PAL-related morbidity was limited (no perioperative complications; a 10.7% grade 3 morbidity rate, all resulting from lymphocysts; and one case of grade 4 morbidity, resulting from acute renal failure, with complete recovery). Lymphocyst formation is a common complication of PAL that is asymptomatic most of the time [21, 23]. In the largest series by Leblanc et al. [6], 14 of their first 104 patients (13.4%) developed symptomatic lymphocysts (12 were managed with either ultrasound- or CT-guided drainage without sequelae and two required reoperation, one by laparoscopy and one by an open procedure). They subsequently performed preventive marsupialization of the left paracolic gutter in the following 77 patients. Among those patients, only three (3.8%) developed a symptomatic lymphocyst. Based on their experience, we performed preventive marsupialization in most patients in our series. This did not completely prevent the formation of symptomatic lymphocysts (one of the three patients requiring reoperation had undergone preventive marsupialization during the initial laparoscopy). All the three reoperation procedures were performed by laparoscopy. These moderate complications did not generate any significant difference in the start of CRT between patients with and without morbidity, which seems essential in this context. To guarantee this acceptable morbidity rate, surgery should be performed by a trained team, and a learning curve is necessary, but with mentoring (companionship) to avoid a high complication rate. For example, in our series, the mean operating times were 227 minutes for the first half of the patients and 186 minutes for the second half of the patients.
The rate of false-negative PET–CT results in our series was 8.4%, which is consistent with the literature [9, 10, 20, 22]. Moreover, half of the false-negative cases had metastases >5 mm. PET–CT, which is the most sensitive imaging method for nodal metastases, cannot replace surgical staging.
In conclusion, PAL seems to generate limited morbidity, but it must be performed by an experienced team to minimize the risks of this procedure, whose prognostic impact appears to be important even though its therapeutic impact is still debated. Less than 4% of the major morbidity could be tolerated, excluding lymphocysts, which can be easily managed, especially with trained radiologists. CRT is the standard treatment and should not be delayed as a result of surgical complications.
Acknowledgments
We would like to thank Lorna Saint Ange for editing.
Author Contributions
Conception/Design: Philippe Morice, Catherine Uzan, Sebastien Gouy, Christine Haie-Meder
Provision of study material or patients: Philippe Morice, Amine Souadka, Catherine Uzan, Sebastien Gouy, Thierry Debaere, Juliette Duclos, Jean Lumbroso, Christine Haie-Meder
Collection and/or assembly of data: Philippe Morice, Amine Souadka, Catherine Uzan, Sebastien Gouy, Thierry Debaere, Juliette Duclos, Jean Lumbroso, Christine Haie-Meder
Data analysis and interpretation: Philippe Morice, Amine Souadka, Catherine Uzan, Sebastien Gouy, Thierry Debaere, Juliette Duclos, Jean Lumbroso, Christine Haie-Meder
Manuscript writing: Philippe Morice, Catherine Uzan, Sebastien Gouy, Christine Haie-Meder
Final approval of manuscript: Philippe Morice, Catherine Uzan, Sebastien Gouy, Christine Haie-Meder
References
- 1.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michel G, Morice P, Castaigne D, et al. Lymphatic spread in stage Ib and II cervical carcinoma: Anatomy and surgical implications. Obstet Gynecol. 1998;91:360–363. doi: 10.1016/s0029-7844(97)00696-0. [DOI] [PubMed] [Google Scholar]
- 3.Varia MA, Bundy BN, Deppe G, et al. Cervical carcinoma metastatic to para-aortic nodes: Extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42:1015–1023. doi: 10.1016/s0360-3016(98)00267-3. [DOI] [PubMed] [Google Scholar]
- 4.Rotman M, Pajak TF, Choi K, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79–20. JAMA. 1995;274:387–393. [PubMed] [Google Scholar]
- 5.Querleu D, Dargent D, Ansquer Y, et al. Extraperitoneal endosurgical aortic and common iliac dissection in the staging of bulky or advanced cervical carcinomas. Cancer. 2000;88:1883–1891. [PubMed] [Google Scholar]
- 6.Leblanc E, Narducci F, Frumovitz M, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304–311. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Grigsby PW. Role of PET in gynecologic malignancy. Curr Opin Oncol. 2009;21:420–424. doi: 10.1097/CCO.0b013e32832ec63f. [DOI] [PubMed] [Google Scholar]
- 8.Kidd EA, Siegel BA, Dehdashti F, et al. Lymph node staging by positron emission tomography in cervical cancer: Relationship to prognosis. J Clin Oncol. 2010;28:2108–2113. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
- 9.Boughanim M, Leboulleux S, Rey A, et al. Histologic results of para-aortic lymphadenectomy in patients treated for stage IB2/II cervical cancer with negative [18F]fluorodeoxyglucose positron emission tomography scans in the para-aortic area. J Clin Oncol. 2008;26:2558–2561. doi: 10.1200/JCO.2007.14.3933. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez PT, Jhingran A, Macapinlac HA, et al. Laparoscopic extraperitoneal para-aortic lymphadenectomy in locally advanced cervical cancer 1: A prospective correlation of surgical findings with positron emission tomography/computed tomography findings. Cancer. 2011;117:1928–1934. doi: 10.1002/cncr.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Dargent D, Ansquer Y, Mathevet P. Technical development and results of left extraperitoneal laparoscopic paraaortic lymphadenectomy for cervical cancer. Gynecol Oncol. 2000;77:87–92. doi: 10.1006/gyno.1999.5585. [DOI] [PubMed] [Google Scholar]
- 13.GEC ESTRO Working Group. Pötter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kupets R, Thomas GM, Covens A. Is there a role for pelvic lymph node debulking in advanced cervical cancer? Gynecol Oncol. 2002;87:163–170. doi: 10.1006/gyno.2002.6815. [DOI] [PubMed] [Google Scholar]
- 16.Delpech Y, Haie-Meder C, Rey A, et al. Para-aortic involvement and interest of para-aortic lymphadenectomy after chemoradiation therapy in patients with stage IB2 and II cervical carcinoma radiologically confined to the pelvic cavity. Ann Surg Oncol. 2007;14:3223–3231. doi: 10.1245/s10434-007-9526-1. [DOI] [PubMed] [Google Scholar]
- 17.Lai CH, Huang KG, Hong JH, et al. Randomized trial of surgical staging (extraperitoneal or laparoscopic) versus clinical staging in locally advanced cervical cancer. Gynecol Oncol. 2003;89:160–167. doi: 10.1016/s0090-8258(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 18.Gold MA, Tian C, Whitney CW, et al. Surgical versus radiographic determination of para-aortic lymph node metastases before chemoradiation for locally advanced cervical carcinoma: A Gynecologic Oncology Group study. Cancer. 2008;112:1954–1963. doi: 10.1002/cncr.23400. [DOI] [PubMed] [Google Scholar]
- 19.Morice P, Bentivegna E, Uzan C, et al. In reply to “Value of positron emission tomography of the para-aortic lymph nodes in cervical carcinoma stage IB2-IIIB“. J Clin Oncol. 2008;26:5655–5657. doi: 10.1200/JCO.2008.19.5883. [DOI] [PubMed] [Google Scholar]
- 20.Leblanc E, Gauthier H, Querleu D, et al. Accuracy of 18-fluoro-2-deoxy-D-glucose positron emission tomography in the pretherapeutic detection of occult para-aortic node involvement in patients with a locally advanced cervical carcinoma. Ann Surg Oncol. 2011 Feb 23; doi: 10.1245/s10434-011-1583-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Tillmanns T, Lowe MP. Safety, feasibility, and costs of outpatient laparoscopic extraperitoneal aortic nodal dissection for locally advanced cervical carcinoma. Gynecol Oncol. 2007;106:370–374. doi: 10.1016/j.ygyno.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Mortier DG, Stroobants S, Amant F, et al. Laparoscopic para-aortic lymphadenectomy and positron emission tomography scan as staging procedures in patients with cervical carcinoma stage IB2-IIIB. Int J Gynecol Cancer. 2008;18:723–729. doi: 10.1111/j.1525-1438.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 23.Marnitz S, Köhler C, Roth C, et al. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol Oncol. 2005;99:536–544. doi: 10.1016/j.ygyno.2005.07.005. [DOI] [PubMed] [Google Scholar]