Data and ongoing research on new cytotoxic and targeted therapies for the treatment of patients with metastatic breast cancer are outlined, and new developments regarding approved but relatively new classes of cytotoxic and targeted agents and also new classes of targeted therapy that are undergoing clinical evaluation are highlighted.
Keywords: Metastatic breast cancer, Combination therapy, Targeted therapy, Monoclonal antibodies, Small-molecule inhibitors
Abstract
Newer treatments have improved survival for patients with metastatic breast cancer over the last two decades, and a battery of new cytotoxic and targeted therapies is continuing to enhance this trend. This review outlines recent data and ongoing research in this area, by highlighting new developments (regarding approved but relatively new classes of cytotoxic and targeted agents) and also new classes of targeted therapy that are undergoing clinical evaluation. Mechanisms for synergy between agents are discussed where data are available, as is information on the rationale behind the development of agents that inhibit angiogenesis, DNA repair, histone deacetylases, heat shock proteins, or various signaling pathways in tumor proliferation. The abundance of clinical research surrounding anticancer agents, together with ongoing cancer biology research, is expected to further increase the available pool of therapeutic options for metastatic breast cancer. Concomitantly, in the absence of an effective targeted monotherapy, a better understanding of the interplay between biologic and cytotoxic anticancer agents will improve our ability to rationally design combination regimens with better efficacy and tolerability.
Introduction
Among women, breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer deaths, claiming about 40,000 lives in North America during 2009 [1]. Recent advancements in targeted therapy and improvements in treatment have heralded a significant improvement in survival [2], but metastatic breast cancer (MBC) remains essentially incurable, with a median 5-year survival rate from diagnosis of only about 23% [3]. As such, the medical community continues its search for novel cytotoxic and biologic approaches to treatment.
Recent advances in our understanding of the molecular pathways underlying oncogenesis and tumor survival have not only enhanced our use of existing agents but also have yielded a collection of novel biologic and cytotoxic agents that are currently under investigation in the treatment of breast cancer. Although we recognize the significance of endocrine manipulation in the MBC treatment setting, this review instead highlights the treatment of human epidermal growth factor receptor (HER-2)+ and triple-negative MBC with investigational combinations of cytotoxic and targeted agents. For a comprehensive discussion of the treatment setting for patients with hormone receptor–positive breast cancer, the reader is advised to review the topic as previously published [4–6].
Novel Cytotoxic Chemotherapy
Several biologic agents have been shown to substantially improve the clinical benefit of standard cytotoxic agents, and these regimens have become the standard of care in both the metastatic and adjuvant settings [6]. To date, however, few targeted biologic therapies have demonstrated efficacy as monotherapies in lieu of cytotoxic chemotherapy [7], making chemotherapy the backbone of MBC treatment. Moreover, alternative cytotoxic strategies are necessary because of greater exposure to standard chemotherapy agents (such as anthracyclines and taxanes) in the adjuvant setting, resulting in less efficacy in the treatment of MBC [8].
Intrinsic or acquired resistance to one or more anticancer agents has been linked to multiple proposed mechanisms, including increased drug efflux (e.g., through overexpression of P-glycoprotein or the multidrug resistance gene), drug inactivation, alterations in the extracellular or intracellular drug target, processing of drug-induced damage, and evasion of apoptosis [9, 10]. In some cases, drug resistance may be a manifestation of tumor survival responses mediated through various cellular mechanisms [11]. Preclinical evidence suggests that hypoxia, glucose deprivation, or possibly other cellular stress stimuli in the tumor microenvironment may boost expression of the class III isoform of tubulin β-3 chain (TUBB3) and decrease expression of other β-tubulin isoforms to which taxanes can bind [11, 12]. Additionally, potential involvement of multiple cell survival pathways and differences in intratumoral drug delivery and concentration are some of the possible reasons behind an observed clinical trend: patients who had previous success with an agent in one class may have an inconsistent response rate to newer agents in the same class (e.g., nanoparticle albumin-bound [nab]-paclitaxel, paclitaxel poliglumex, and larotaxel in the taxane class [13] and vinflunine [14] in the vinca alkaloid class).
The cytotoxic treatments most commonly used after anthracycline and/or taxane failure are often those with disparate mechanisms of action, such as capecitabine or gemcitabine [6]. However, in the case of taxanes, newer generations of microtubulin-targeting agents (i.e., epothilones and halichondrins) have provided other options for treatment. Epothilones have a mechanism of action similar to that of taxanes but are less susceptible than taxanes to P-glycoprotein–mediated efflux, tubulin mutation, and overexpression of TUBB3 [15]. Halichondrins bind and sequester β-tubulin into nonfunctional aggregates [16]. In the subsequent section, we discuss members of these two newer classes of tubulin-targeting agents: epothilones (ixabepilone, patupilone, and sagopilone) and halichondrins (eribulin) for the treatment of taxane-resistant MBC.
Ixabepilone and eribulin each have demonstrated efficacy in patients with MBC who have been previously treated with anthracyclines and taxanes. Ixabepilone is approved (in combination with capecitabine) by the U.S. Food and Drug Administration (FDA) for the treatment of patients with MBC. In a phase III registration trial, this combination offered greater clinical efficacy than capecitabine alone for the primary endpoint of progression-free survival (PFS) (hazard ratio [HR], 0.75; 95% confidence interval [CI], 0.64–0.88; p = .0003). Furthermore, the median PFS interval was longer, at 5.8 months (95% CI, 5.45–6.97 months) for the combination, compared with 4.2 months (95% CI, 3.81–4.50 months) for capecitabine alone [17]. Based on phase II data, ixabepilone is also approved as monotherapy in patients with MBC resistant to taxanes, anthracyclines, and capecitabine [18], and additional studies are ongoing. Novel cytotoxic agents, including ixabepilone, are currently being investigated in combination with a variety of established and investigational biologic agents (including monoclonal antibodies, small molecule kinase inhibitors, and histone deacetylase [HDAC] inhibitors).
In the phase III Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus Eribulin E7389 (EMBRACE) in patients with MBC previously treated with an anthracycline and a taxane, eribulin led to a significantly longer overall survival (OS) time than with the physician's choice as salvage therapy (13.1 months versus 10.7 months; HR, 0.81; 95% CI, 0.66–0.99; p = .041) [19]. A higher overall response rate (ORR) was demonstrated in patients treated with eribulin than in those treated with the physician's choice, confirmed by both independent review (12.2% versus 4.7%; p = .002) and investigator assessment (13.2% versus 7.5%; p = .028). However, a longer PFS duration was shown (median, 3.6 months versus 2.2 months; HR, 0.76; 95% CI, 0.64–0.90; p = .002), but this was found to be not statistically significant by the independent reviewers (median, 3.7 months versus 2.2 months; HR, 0.87; 95% CI, 0.71–1.05; p = .14). Notably, the EMBRACE trial is the first phase III, single-agent trial in heavily pretreated patients with MBC to show a significant improvement in OS, thus leading to FDA approval of this drug in late 2010.
Targeted Therapies
Anti–HER-2 Therapy
Synergy of Trastuzumab and Cytotoxic Combinations
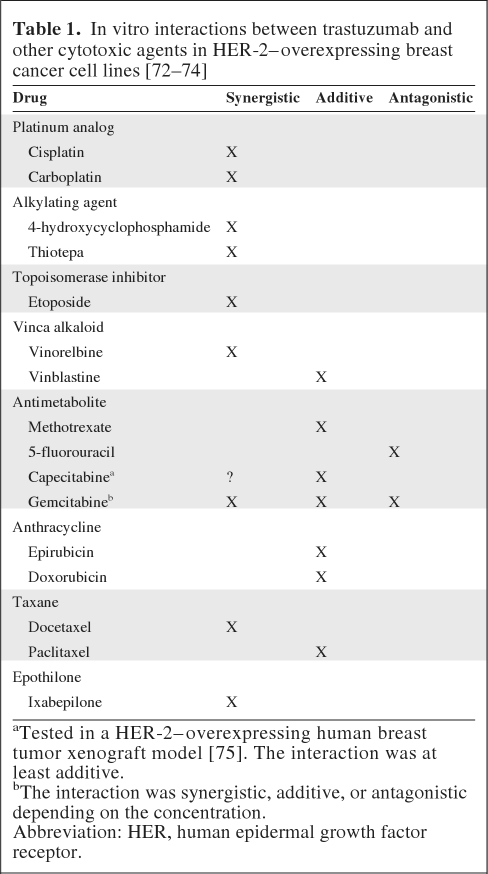
HER-2 is amplified and/or overexpressed in up to 30% of human breast tumors and is associated with a poorer prognosis [20, 21]. A new generation of HER-2–targeting agents has been developed and alternative cytotoxic combination strategies are currently being explored. One such HER-2–targeting agent, trastuzumab, has demonstrated synergistic activity against breast cancer cells overexpressing HER-2 when used in combination with cisplatin, carboplatin, vinorelbine, gemcitabine, ixabepilone, and docetaxel in the preclinical setting (Table 1). Further to these findings, a pivotal phase III study of women whose cancers overexpressed HER-2 and were chemotherapy naive in the metastatic setting demonstrated that the combination of trastuzumab and first-line chemotherapy was associated with a significantly longer time to progression (TTP) (median, 7.4 months versus 4.6 months; p < .001), a higher ORR (50% versus 32%; p < .001), a longer duration of response (DoR) (median, 9.1 months versus 6.1 months; p < .001), and a longer OS time (median, 25.1 months versus 20.3 months; p = .046) [22].
Table 1.
In vitro interactions between trastuzumab and other cytotoxic agents in HER-2–overexpressing breast cancer cell lines [72–74]
aTested in a HER-2–overexpressing human breast tumor xenograft model [75]. The interaction was at least additive.
bThe interaction was synergistic, additive, or antagonistic depending on the concentration.
Abbreviation: HER, human epidermal growth factor receptor.
In clinical practice, trastuzumab is routinely used with combination chemotherapy in the treatment of breast cancer patients in both the adjuvant and metastatic settings [6]. In the first-line MBC setting, when compared with docetaxel alone, trastuzumab in combination with docetaxel resulted in a significantly greater ORR (61% versus 34%; p = .0002), longer OS time (median, 31.2 months versus 22.7 months; p = .0325), longer TTP (median, 11.7 months versus 6.1 months; p = .0001), longer time to treatment failure (median, 9.8 months versus 5.3 months; p = .0001), and longer DoR (median, 11.7 months versus 5.7 months; p = .009) [23]. In an effort to further the clinical benefit of combinatorial trastuzumab and nonanthracycline agents, a phase III trial evaluated the efficacy and safety of trastuzumab in combination with paclitaxel with or without carboplatin as first-line therapy for women with HER-2–overexpressing MBC. In that study, the addition of carboplatin to paclitaxel and trastuzumab resulted in significantly better clinical outcomes, with an ORR of 57% (95% CI, 45%–70%), versus 36% (95% CI, 25%–48%; p = .03), and a median PFS interval of 13.8 months, versus 7.6 months (p = .05) for the combination, with or without carboplatin (HR, 0.55; 95% CI, 0.46–0.64) [24]. However, in the randomized phase III Breast Cancer International Research Group 007 trial, a study that investigated the efficacy and safety of trastuzumab in combination with docetaxel with or without carboplatin as first-line therapy for women with HER-2–overexpressing MBC, there were no significant differences observed between treatment arms with respect to the TTP (median, 10.4 months and 11.1 months; HR, 0.914; 95% CI, 0.694–1.203; p =.57), response rate (72% for both arms), or OS time (median, 37.4 months versus 37.1 months; p = .99) [25]. Together, these studies suggest some level of ambiguity surrounding the efficacy of trastuzumab in combination with a taxane and carboplatin as first-line treatment for HER-2–overexpressing MBC.
Today, trastuzumab continues to undergo clinical development in combination with several newer cytotoxic agents, including nab-paclitaxel and ixabepilone. A trial of first-line nab-paclitaxel, trastuzumab, and carboplatin demonstrated a 46% ORR and a median TTP of 16 months [26]. Similarly, a trial of ixabepilone, trastuzumab, and carboplatin as first-line treatment showed an ORR of 41% and median PFS interval of 8 months [27]; a second trial with ixabepilone and trastuzumab showed an ORR of 51% [28].
Notably, if patients fail first-line treatment with combination trastuzumab and chemotherapy, replacing the chemotherapeutic agent with another can facilitate continuation of trastuzumab and better outcomes. As demonstrated in the phase III German Breast Group 26/Breast International Group 03–05 trial, the continuation of trastuzumab in combination with second-line chemotherapy (capecitabine) resulted in a higher ORR (48.1% versus 27.0%; p = .0115) and longer median TTP (8.2 months versus 5.6 months) (HR, 0.69; 95% CI, 0.48–0.97; p = .0338) when compared with capecitabine alone [29]. Alternatively, patients may be treated with lapatinib—a small-molecule inhibitor of both the HER-2 and epidermal growth factor receptor (EGFR) pathways (as discussed below). In combination with capecitabine, lapatinib is the standard approved therapy for the treatment of patients with advanced breast cancer or MBC whose tumors overexpress HER-2 and who have received prior therapy including an anthracycline, a taxane, and trastuzumab [30].
The Newer Generation of HER-2–Targeting Monoclonal Antibodies
Trastuzumab-MCC-DM1.
The additive or synergistic potential of trastuzumab, in combination with microtubule-targeting agents, has resulted in trastuzumab-MCC-DM1 (T-DM1)—a novel antibody–drug conjugate that uses trastuzumab to specifically deliver the cytotoxic maytansinoid antimicrotubule to HER-2+ cells. Mechanistically, DM1 binds to microtubules in a manner similar to that of vinca alkaloids [31].
The first-in-human phase I, multicenter, open-label, dose-escalation study of single-agent T-DM1 in patients with HER-2+ MBC (who had previously received a trastuzumab-containing chemotherapy regimen) demonstrated that, at the maximum-tolerated dose (MTD) of 3.6 mg/kg every 3 weeks, T-DM1 was safe and had considerable clinical activity. The clinical benefit rate (CBR [ORR plus stable disease (SD) at 6 months]) among 15 patients treated at the MTD was 73%, including five objective responses. Furthermore, the confirmed response rate in patients with measurable disease at the MTD (n = 9) was 44% [31]. Phase II studies of T-DM1 in patients with HER-2+ MBC who progressed while receiving HER-2–directed therapy, or who were previously treated with an anthracycline, a taxane, capecitabine, lapatinib, and trastuzumab, have demonstrated robust activity, with ORRs in the range of 23.9%–39.5%, as determined by an independent-review facility [32, 33]. An open-label, phase III trial (EMILIA) will compare the safety and efficacy of T-DM1 with that of capecitabine in combination with lapatinib in patients with HER-2+ MBC previously treated with a trastuzumab-based therapy [34]. Currently, T-DM1 is in clinical development for the treatment of MBC as both a monotherapy and in combination with other agents such as docetaxel or pertuzumab plus paclitaxel.
Pertuzumab.
Pertuzumab is a novel recombinant humanized monoclonal antibody directed against the highly conserved dimerization domain of HER-2, and as such, it inhibits HER-2 homo- and heterodimerization. Pertuzumab-mediated blockage of HER-2 dimerization inhibits HER family downstream signaling (i.e., the Akt cell survival pathway and the mitogen-activated protein kinase pathway). Preclinical models have demonstrated that, unlike trastuzumab, pertuzumab is able to inhibit tumor growth of cancer cells expressing both low/normal and high levels of HER-2. However, in a phase II randomized trial investigating the efficacy and safety of pertuzumab in patients with HER-2− MBC, the only measurable therapeutic benefit that Gianni and colleagues observed was with SD of a relatively short duration. These data suggest that patients with HER-2+ MBC would be more likely to experience a clinically meaningful response from treatment with pertuzumab [35]. The idea that the combination of pertuzumab and T-DM1 might be a clinically meaningful therapy in MBC came from the single-arm, phase II trial of trastuzumab plus pertuzumab, which demonstrated that the combination was well tolerated and active in patients with HER-2+ MBC who had progressed during trastuzumab therapy [36]. Ongoing clinical trials continue to investigate pertuzumab for the treatment of MBC in combination with trastuzumab, T-DM1, docetaxel, and paclitaxel. Because preclinical evidence suggests resistance may, in many cases, arise from aberrant signaling downstream of HER-2 (e.g., constitutive phosphoinositide 3-kinase [PI3K] signaling), it is not yet clear whether pertuzumab will be effective in trastuzumab-resistant tumors [37].
Agents Targeting EGFR Pathways
Resistance to trastuzumab has necessitated a search for other agents that use the EGFR signaling pathways. Although oncologists and researchers continue to debate the relative merits of monoclonal antibodies and small-molecule inhibitors [38], an understanding of how each works in various patient populations may help maximize optimal clinical outcomes; both types of targeted therapy have demonstrated efficacy in clinical trials and are undergoing further evaluation in MBC [38].
Cetuximab
EGFR is often overexpressed in breast cancer, particularly in triple-negative breast cancer. In the phase II TBCRC (Translational Breast Cancer Research Consortium) 001 trial, the EGFR inhibitor cetuximab was shown to have limited activity as monotherapy in a triple-negative MBC population treated with three or fewer prior chemotherapies (two of 31 [6%] patients achieved partial responses, with a CBR of 10%). However, when used in combination with carboplatin, an ORR of 18% (13 of 17 patients) was observed, with a higher CBR of 27% [39]. Although cetuximab monotherapy was well tolerated, it appears to have low activity in the triple-negative MBC population and thus, as a monotherapy, would not be recommended for treating this tumor subtype. Several phase II studies in patients with triple-negative MBC are investigating combinations of cetuximab with platinum agents. Data from the ongoing phase II trial of cetuximab with and without cisplatin for the treatment of triple-negative MBC (BALI-1 [Basal like]) presented at the 2010 European Society for Medical Oncology (ESMO) Congress showed that the combination was effective in women with triple-negative disease, with a significantly lower risk for progression than with cisplatin alone (HR, 0.675; p = .032) [40]. Notably, the median PFS time was more than double. Although there was a trend toward a higher tumor response rate with cetuximab plus cisplatin (20.0% versus 10.3%; p = .11), the primary endpoint (response rate) was not met. Cetuximab in combination with ixabepilone is also under investigation as a therapeutic option for the first-line treatment of patients with triple-negative MBC.
Small-Molecule Inhibitors of the HER-2 and EGFR Pathways
Lapatinib
Lapatinib inhibits intracellular receptor phosphorylation of HER-2 and EGFR [41, 42], whereas trastuzumab targets the extracellular domains of HER-2. These distinct mechanisms of action may account for the lack of crossresistance between trastuzumab and lapatinib and for their in vitro synergy [42]. In a phase III trial of lapatinib in combination with trastuzumab compared with lapatinib alone in 296 heavily pretreated patients with HER-2+ MBC who had experienced progression on a median of three prior trastuzumab-based therapies (the EGF104900 trial), the PFS interval was significantly longer with the combination than with lapatinib alone (median, 3.0 months versus 2.0. months; HR, 0.77; 95% CI, 0.6–1.0; p = .029). However, there was no significant difference in the ORR (10.3% versus 6.9%; p = .46), and although there was a trend toward a longer OS duration for patients in the combination arm, this difference was also not significant (12.9 months versus 9.8 months; HR, 0.75; 95% CI, 0.5–1.1; p = .106) [43]. Notably, these mature OS data are not in agreement with what was previously reported at the 2009 San Antonio Breast Cancer Symposium (SABCS), where Blackwell and colleagues observed a significantly longer OS time in patients treated with combination therapy than in those treated with lapatinib monotherapy (HR, 0.74; 95% CI, 0.57–0.97; p = .026) [44].
Thus far, discussion of lapatinib in the treatment of HER-2+ breast cancer has been limited to the metastatic setting. However, the clinical benefit of neoadjuvant lapatinib is also being investigated. Results from the phase III trial of neoadjuvant lapatinib with or without trastuzumab treatment optimization (NeoALTTO [companion trial to the larger ALTTO study]) were presented at the 2010 SABCS [45]. NeoALTTO enrolled 455 patients who were randomized to lapatinib (1,500 mg/day), trastuzumab (4 mg/kg i.v. loading dose followed by 2 mg/kg i.v. weekly), or lapatinib (1,000 mg/day) in combination with the same dose of trastuzumab for 6 weeks. Paclitaxel was added to all three arms at week 6, for a total of 12 weeks of neoadjuvant therapy. The primary endpoint of the NeoALTTO trial was pathologic complete response (pCR). In the combination arm, 51.3% of patients experienced a pCR, compared with 24.7% of patients in the trastuzumab arm and 19.5% of patients in the lapatinib arm (p = .0001 for the difference between the combination arm and the trastuzumab arm; the difference between the lapatinib and trastuzumab groups was not statistically significant). The benefit of the combination of lapatinib and trastuzumab was more pronounced in hormone receptor–negative patients than in hormone receptor–positive patients (61.3% versus 41.6%, respectively). The difference in pCR rates between the combination arm and the trastuzumab arm was significant in both groups, based on hormone receptor status: p = .005 in the hormone receptor–negative group and p = .03 in the hormone receptor–positive group. These preliminary data from the NeoALTTO trial demonstrate the synergistic potential of lapatinib and trastuzumab combination therapy. Although there was more toxicity associated with lapatinib, it was found to be manageable.
Neratinib
Neratinib, which irreversibly inhibits the kinase activity of HER-2 and EGFR, showed a 32% response rate as a single agent in trastuzumab-pretreated MBC patients [46]. As such, this agent is currently being studied in combination with capecitabine, paclitaxel, or vinorelbine in phase I/II trials of patients with HER-2+ MBC. Moreover, a large randomized phase II trial designed to evaluate the safety and efficacy of neratinib in advanced HER-2+ breast cancer is ongoing (ClinicalTrials.gov identifier, NCT00300781). The estimated enrollment is 136 patients and the primary endpoint is the 16-week PFS rate.
Antiangiogenic Therapy
Bevacizumab
Bevacizumab is a humanized monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), depriving tumors of a vascular supply and inhibiting endothelial proliferation [47]. In a prospective, randomized, but not blinded phase III trial in the first-line treatment of MBC patients, the combination of bevacizumab and paclitaxel led to a significantly longer PFS interval than with paclitaxel alone (median, 11.8 months versus 5.9 months; HR, 0.60; p < .001) and higher ORR (36.9% versus 21.2%; p < .001) with minimal toxicity, not unlike safety profiles reported in previous randomized trials [47]. However, there was no significant difference in OS between the groups (median, 26.7 months versus 25.2 months; HR, 0.88; p = .16). Additional confirmatory trials combining this agent with docetaxel (AVADO [Avastin and docetaxel]) [48] or with anthracyclines and taxanes or capecitabine (RIBBON-1 [Regimens in Bevacizumab for Breast Oncology]) have demonstrated enhanced efficacy in the first-line setting [49].
In the AVADO trial, the addition of bevacizumab to docetaxel resulted in a longer PFS time (7.5 mg/kg: HR, 0.69; 95% CI, 0.54–0.89; p = .0035; 15 mg/kg: HR, 0.61; 95% CI, 0.48–0.78; p < .0001) and greater ORR (7.5 mg/kg: 44.4% versus 55.2%; p = .00295; 15 mg/kg: 44.4% versus 63.1%; p < .0001). Similarly, results from the RIBBON-1 trial demonstrated that the addition of bevacizumab to capecitabine or to taxane-/anthracycline-based chemotherapy led to a longer PFS time (HR, 0.69; 95% CI, 0.56–0.84; p = .0002; HR, 0.64; 95% CI, 0.52–0.80; p < .0001, respectively) and higher ORR (35.4% versus 23.6%; p = .0097; 51.3% versus 37.9%; p = .0054, respectively). However, the addition of bevacizumab to chemotherapy did not result in a longer OS time in either the AVADO or RIBBON-1 trial.
A meta-analysis of OS from the phase III randomized trial evaluating bevacizumab in combination with paclitaxel (E2100) and the AVADO and RIBBON-1 trials (a total of 2,447 patients) confirmed the significant PFS benefit for bevacizumab in combination with chemotherapy, compared with chemotherapy alone for the first-line treatment of MBC patients (median, 9.2 months versus 6.7 months; HR, 0.64; p < .0001) [50]. Although the OS time was not significantly different between treatment arms (median, 26.7 months versus 26.4 months; HR, 0.97; 95% CI, 0.86–1.08; p = .56), the 1-year survival rate was greater for bevacizumab in combination with chemotherapy than for chemotherapy alone (81.6% versus 76.5%; p = .003), suggesting an early benefit at 1 year.
A preliminary efficacy analysis of data from a phase III, multicenter, randomized, placebo-controlled trial that evaluated the efficacy and safety of bevacizumab in combination with chemotherapy regimens in patients with previously treated MBC (RIBBON-2) also demonstrated that the addition of bevacizumab to chemotherapy led to a longer PFS duration than with chemotherapy plus placebo (median, 7.2 months versus 5.1 months; HR, 0.78; 95% CI, 0.64–0.93; p = .0072) [51]. Moreover, an ongoing phase III study is also evaluating bevacizumab in combination with weekly schedules of ixabepilone, paclitaxel, or albumin-bound paclitaxel. Despite the PFS benefit shown in the E2100, AVADO, RIBBON-1, and RIBBON-2 trials, the FDA has announced their recommendation to remove the breast cancer indication from the label for bevacizumab, stating that the data do not demonstrate longer OS or a sufficient benefit from bevacizumab in slowing disease progression to outweigh the significant risk to patients with breast cancer. These risks include severe high blood pressure; bleeding and hemorrhage; perforations in the nose, stomach, and intestines; and heart attack or heart failure. Antiangiogenesis in breast cancer remains an active area of interest with ongoing trials in the neoadjuvant and adjuvant settings from cooperative groups (Eastern Cooperative Oncology Group and Cancer and Leukemia Group B). Given the marginal benefit and toxicity of bevacizumab, the VEGF target will need to be defined specifically for each particular subtype of breast cancer to maximize benefits in treated patients.
Multikinase Inhibitors
Sorafenib
Sorafenib is a small-molecule inhibitor that targets all VEGF receptor isoforms, platelet-derived growth factor receptor (PDGFR)-β, several of the Raf family of intracellular kinases, and several other cell surface kinases (Kit, Flt-3, and Ret). In a phase II trial of patients randomized to paclitaxel plus placebo or paclitaxel in combination with sorafenib, sorafenib failed to lead to a significantly longer PFS interval than with placebo (median, 6.9 months versus 5.6 months; HR, 0.89; 95% CI, 0.56–1.11; p = .0857) [52]. However, secondary endpoints of the TTP (median, 8.1 months versus 5.6 months; HR, 0.67; p = .0171) and ORR (68% versus 54%; p = 0.0234) were significantly better with sorafenib. Notably, the toxicities associated with combining sorafenib with chemotherapy can be substantial. For example, a phase II trial of sorafenib in combination with capecitabine demonstrated that the combination resulted in a higher incidence of grade 3 hand–foot syndrome than with capecitabine alone (45% versus 13%) [53]. Phase II trials of sorafenib in combination with other cytotoxic agents, including vinorelbine and ixabepilone, are ongoing.
Sunitinib
Like sorafenib, sunitinib is a small-molecule pan-kinase inhibitor that targets multiple intracellular growth and angiogenic factors. In heavily pretreated MBC patients, sunitinib demonstrated some level of clinical activity as a monotherapy [54] and in combination with docetaxel [55]. Other sunitinib-based combinations currently in phase II study include sunitinib plus paclitaxel, sunitinib plus capecitabine, and, in triple-negative disease, sunitinib plus ixabepilone. Data from two phase III trials evaluating sunitinib in combination with either docetaxel or capecitabine versus each agent alone in previously treated advanced breast cancer patients were presented at the 2010 American Society of Clinical Oncology Annual Meeting. Although both trials showed that combining sunitinib with either docetaxel or capecitabine led to a higher ORR (docetaxel: 55% versus 42%; p = .001; capecitabine: 19% versus 18%), neither combination resulted in a significantly longer PFS interval (sunitinib plus docetaxel: median, 8.6 months versus 8.3 months; HR, 0.92; 95% CI, 0.72–1.19; p = .265; sunitinib plus capecitabine: median, 5.5 months versus 5.9 months; HR, 1.22; 95% CI, 0.95–1.58; p = .941) or OS time (sunitinib plus docetaxel: median, 24.8 months versus 25.5 months; HR, 1.21; 95% CI, 0.91–1.60; p = .904; sunitinib plus capecitabine: median, 16.4 months versus 16.5 months; HR, 0.99; 95% CI, 0.76–1.30; p = .484) than with single-agent docetaxel or capecitabine [56, 57]. Thus, these regimens are not recommended for the treatment of patients with advanced MBC.
Imatinib
Imatinib specifically targets PDGFR-α and PDGFR-β, c-Kit, and Bcr-Abl. A phase II trial of imatinib plus capecitabine failed to show any difference in efficacy compared with capecitabine alone in patients who had already received at least two lines of metastatic treatment [58]. Imatinib is currently undergoing further phase II evaluations with either vinorelbine, docetaxel, or gemcitabine.
Targeting Novel Molecular Pathways
Poly(ADP Ribose) Polymerase Inhibitors
The clinical efficacy of poly(ADP ribose) polymerase (PARP) inhibitors is attributed to their ability to take advantage of the impaired mechanism of double-strand break (DSB) repair used by some tumors. DNA lesions such as single-strand breaks (SSBs) and DSBs are common byproducts of normal cellular metabolism, and may also result from exposure to harmful environmental agents. In brief, four DNA repair mechanisms are responsible for repairing these lesions: (a) base-excision repair (BER), (b) nucleotide-excision repair (NER), (c) mismatch repair (MMR), and (d) recombinational repair (with homologous recombination and nonhomologous end joining [NHEJ]) [59]. When SSBs occur, they are repaired using the intact complementary strand as a template by BER, NER, and MMR. A key component of the BER pathway, PARP1 is the most important member of the PARP family of enzymes.
DSBs are primarily repaired by homologous recombination—almost error free—and NHEJ, which is more susceptible to error. The tumor suppressor proteins BRCA1 and BRCA2 play a pivotal role in the repair of DSBs by homologous recombination. However, in BRCA-deficient cells that have accumulated DSBs, loss of BRCA function renders the cell incapable of repairing DSBs by homologous recombination. Instead, the cell defaults to the error-prone NHEJ pathway, resulting in genomic instability that ultimately leads to cell death. Thus, patients whose tumors are BRCA deficient are ideal candidates for treatment with PARP inhibitors in the clinical setting. Because triple-negative breast cancer shares pathologic and gene expression profiles with BRCA-associated breast cancers, both subtypes are currently being evaluated in clinical trials of PARP inhibitors—as single agents and in combination with chemotherapy.
In a phase II trial of heavily pretreated patients with recurrent, measurable, chemotherapy-refractory BRCA mutation–associated breast cancer, the PARP inhibitor olaparib (400 mg twice daily and subsequently 100 mg twice daily) demonstrated measurable single-agent efficacy, with an ORR as high as 41% [60]. Like BRCA-associated breast cancers, triple-negative breast cancer is sensitive to PARP inhibition in the presence of platinum-based chemotherapy.
In the first randomized phase II trial of a PARP inhibitor in triple-negative MBC patients, iniparib (5.6 mg/kg i.v. biweekly) on days 1, 4, 8, and 11 every 3 weeks was combined with chemotherapy (gemcitabine, 1,000 mg/m2, and carboplatin, area under the curve [AUC] = 2, both given on days 1 and 8 every 3 weeks) to determine the effect on PFS and OS. The addition of iniparib to gemcitabine plus carboplatin not only resulted in a longer PFS duration (median, 7.0 months versus 2.9 months; HR, 0.30; 95% CI, 0.15–0.59; p = .0003) but also resulted in a longer OS time (median, >8.5 months versus 5.6 months; HR, 0.24; 95% CI, 0.09–0.61; p = .0012) and higher CBR (52% versus 12%; p = .0012) than with gemcitabine plus carboplatin alone [61]. Updated results presented at the 2010 ESMO Congress showed that, with extended follow-up, the combination led to a significantly longer OS time than with gemcitabine plus carboplatin alone (12.2 months versus 7.7 months; HR, 0.5; 95% CI, 0.30–0.82; p = .005) [59]. A phase III, randomized, multicenter trial of gemcitabine plus carboplatin with or without iniparib in patients with triple-negative breast cancer is now complete and awaits assessment (ClinicalTrials.gov identifier, NCT00938652). Importantly, on January 28, 2011, a press release from Sanofi-Aventis and its subsidiary BiPar Sciences stated that this phase III trial did not meet the prespecified criteria for significance for the coprimary endpoints of OS and PFS. However, data from the prespecified analysis in patients treated in the second- and third-line setting do support the findings reported in the phase II trial—longer OS and PFS times. With data to support the efficacy of PARP inhibitors in the clinical setting, researchers have begun to focus on tailoring treatment regimens according to specific triple-negative subtypes.
A translational phase II trial of olaparib in patients with ovarian cancer (BRCA1 and BRCA2 carriers) and triple-negative breast cancer (BRCA1 and BRCA2 noncarriers) demonstrated that, as a single agent, olaparib provided clinical benefit only to patients with ovarian cancer [62]. In that study, the ORR was approximately 41.2% in 64 women with ovarian cancer, whereas the ORR was zero in 24 patients with triple-negative MBC. These data suggest that triple-negative breast cancer BRCA1 and BRCA2 carriers may be better candidates for therapy with single-agent PARP inhibitors than triple-negative breast cancer BRCA1 and BRCA2 noncarriers.
Because BRCA1-associated triple-negative tumors are more likely to have a deficiency in DNA repair than sporadic triple-negative tumors, Rodriguez and colleagues [63] designed a study to define a gene-expression signature that would facilitate their identification. Such an assay could enable the identification of patients with triple-negative breast cancer who would have a greater response to neoadjuvant DNA-damaging agents than patients with a different expression profile.
From their investigations, a defective DNA repair gene-expression signature of 334 genes was derived, and from this signature a subset of 69 of the most differentially expressed genes was confirmed. After testing the association of this signature with pathologic response in neoadjuvant trials of 5-fluorouracil, epirubicin, and cyclophosphamide; doxorubicin plus cyclophosphamide; and taxane-based epirubicin plus docetaxel chemotherapy, the investigators found that this defective DNA repair microarray gene-expression pattern was significantly associated with anthracycline response (AUC, 0.61; 95% CI, 0.45–0.77) and taxane resistance (AUC, 0.65; 95% CI, 0.46–0.85). The clinical applicability of this defective DNA repair gene-expression signature and use with PARP1 inhibitors is readily apparent, and it is thus a likely candidate for further clinical development.
Mammalian Target of Rapamycin Inhibitors
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase responsible for regulating cell proliferation, survival, and transcription. A study from Nahta and Esteva suggested that inhibiting mTOR may circumvent trastuzumab resistance mediated by aberrant PI3K activity [37]. Everolimus, an oral mTOR inhibitor, has been shown to enhance the efficacy of trastuzumab and partially reverse resistance [64]. A multicenter, phase I trial of daily and weekly everolimus in combination with vinorelbine and trastuzumab showed promising anticancer activity in patients with HER-2–overexpressing, trastuzumab-resistant MBC [65]. This regimen is now in phase III evaluation. In the same setting, a phase II trial is also investigating deforolimus—another mTOR inhibitor—in combination with trastuzumab.
HDAC Inhibitors
Epigenetic changes associated with oncogenesis and progression occur through a variety of mechanisms, including changes in the degree of association between DNA and histone proteins. Histone acetyltransferases and HDACs regulate the dynamic equilibrium between histone acetylation and deacetylation, which in turn modulates DNA transcription [66]. Inhibiting HDACs may enhance the transcription of genes that mediate cell-cycle arrest, a mechanism that is at least partially responsible for HDAC inhibitor–mediated cell death [66, 67].
Vorinostat (suberoylanilide hydroxamic acid) is a small-molecule inhibitor of both class I and class II HDAC enzymes [67]. Class I and class II HDAC enzymes differ in their expression patterns; HDACs of class I are expressed in most cell types, whereas the expression pattern of class II HDACs is more restricted [68]. Moreover, localization patterns restrict class I HDACs almost exclusively to the nucleus, whereas class II HDACs are capable of shuttling in and out of the nucleus in response to specific cellular signals. As a single agent, vorinostat showed a 29% SD rate in a National Cancer Institute–sponsored phase II trial of 14 patients with MBC [69]. Ongoing trials of vorinostat in combination with paclitaxel and bevacizumab, capecitabine, and ixabepilone (BMS CA163–202) will further establish the efficacy of this treatment strategy in MBC patients.
Panobinostat (LBH589), a related hydroxamic acid, is in phase I/II investigation in combination with trastuzumab and paclitaxel, and with capecitabine and lapatinib for the treatment of patients with MBC. Early preclinical data suggested possible reversal of or delayed resistance to aromatase inhibition in breast cancer. For example, one study reported that panobinostat selectively suppressed human aromatase gene promoters I.3/II, which are preferentially used in breast cancer tissue [70]. Moreover, in combination with letrozole—an aromatase inhibitor—panobinostat synergistically suppressed the in vitro proliferation of hormone-responsive breast cancer cells. As such, this strategy is currently under active investigation for hormone receptor–positive breast cancers.
Heat Shock Protein Inhibitors
Heat shock proteins (HSPs) are some of the most abundant proteins in normal cells. These chaperone molecules function in protein folding and cell signaling, protecting cells from cellular stress–mediated apoptosis. In particular, preclinical data suggest that expression of HSP90 is upregulated in response to cellular stress factors such as those found in the tumor microenvironment, providing a rationale to target HSP90 as an anticancer therapy [71]. To this end, phase II trials are evaluating the efficacy of an HSP90 inhibitor, tanespimycin (17AAG, 17-allylamino-17-demethoxygeldanamycin), as monotherapy in patients with refractory (disease progression after prior treatment with or a contraindication to an anthracycline- and a taxane-based regimen) MBC and with trastuzumab in patients who have progressed after prior trastuzumab therapy. Retaspimycin hydrochloride (IPI-504), another small-molecule HSP90 inhibitor, promotes the degradation of oncogenic signaling proteins, inducing apoptosis. Retaspimycin hydrochloride, in combination with trastuzumab, is currently under investigation in a phase II trial of patients with pretreated, locally advanced or metastatic breast cancer.
Conclusion
Recent additions of novel therapeutics to the breast cancer armamentarium have resulted in the prolongation of survival for patients with MBC. It is hoped that, in the coming years, the number of treatment options for MBC patients will continue to increase. The clinical development of targeted agents as partners with chemotherapy has been supported by data showing better efficacy in terms of the PFS interval, ORR, and, less commonly, OS time. With this strategy, crossresistance remains a concern because targeted and cytotoxic agents may have different but overlapping mechanisms of action. The safety profiles of cytotoxic and biologic compounds are expected to overlap only in limited ways. It is worth noting, however, that the target proteins of biologic agents that are dysregulated in cancer are commonly essential components of normal cell growth and development. Thus, it is challenging to completely anticipate any potential toxicities associated with inhibiting endogenous levels of these proteins and how this inhibition might exacerbate toxicities associated with chemotherapy (i.e., bevacizumab plus cytotoxic chemotherapy).
A better understanding of the molecular interactions between targeted agents and cytotoxic chemotherapy, combined with increasing knowledge of the molecular mechanisms underlying drug resistance, has enabled the rational design of effective combination regimens for the treatment of MBC patients. With these advancements in breast cancer biology, the natural history of MBC now resembles that of a chronic disease for which longer survival and preservation of quality of life are the goals of treatment. The expanding number of possibilities for regimens that combine new anticancer agents may be expected to translate into better outcomes for patients with MBC.
Author Contributions
Conception/Design: Thehang Luu, Cathie Chung, George Somlo
Manuscript writing: Thehang Luu, Cathie Chung, George Somlo
Final approval of manuscript: Thehang Luu, Cathie Chung, George Somlo
The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoint and medical expertise. They also wish to acknowledge StemScientific, funded by Bristol-Myers Squibb Company, for providing writing and editorial support. Neither Bristol-Myers Squibb Company nor StemScientific influenced the content of this manuscript, nor did the authors receive financial compensation for authoring the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Chia SK, Speers CH, D'yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 3.Horner MJ, Ries LAG, Krapcho M, et al. Bethesda, MD: National Cancer Institute; [accessed March 24, 2011]. SEER Cancer Statistics Review, 1975–2006. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/f861009final90809pdf.pdf. [Google Scholar]
- 4.Barrios CH, Sampaio C, Vinholes J, et al. What is the role of chemotherapy in estrogen receptor-positive, advanced breast cancer? Ann Oncol. 2009;20:1157–1162. doi: 10.1093/annonc/mdn756. [DOI] [PubMed] [Google Scholar]
- 5.Guarneri V, Conte P. Metastatic breast cancer: Therapeutic options according to molecular subtypes and prior adjuvant therapy. The Oncologist. 2009;14:645–656. doi: 10.1634/theoncologist.2009-0078. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer - V.I. 2010. [accessed March 24, 2011]. Available at http://www.nccn.org.
- 7.Cavallo J. Can You Afford Cancer? Cure. 2006. [accessed March 24, 2011]. Available at http://www.curetoday.com/index.cfm/fuseaction/article.show/id/2/article_id/365.
- 8.Moreno-Aspitia A, Perez EA. Anthracycline- and/or taxane-resistant breast cancer: Results of a literature review to determine the clinical challenges and current treatment trends. Clin Ther. 2009;31:1619–1640. doi: 10.1016/j.clinthera.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Fojo T, Menefee M. Mechanisms of multidrug resistance: The potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(suppl 5):v3–v8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 10.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 11.Ferlini C, Raspaglio G, Cicchillitti L, et al. Looking at drug resistance mechanisms for microtubule interacting drugs: Does TUBB3 work? Curr Cancer Drug Targets. 2007;7:704–712. doi: 10.2174/156800907783220453. [DOI] [PubMed] [Google Scholar]
- 12.Cicchillitti L, Penci R, Di Michele M, et al. Proteomic characterization of cytoskeletal and mitochondrial class III beta-tubulin. Mol Cancer Ther. 2008;7:2070–2079. doi: 10.1158/1535-7163.MCT-07-2370. [DOI] [PubMed] [Google Scholar]
- 13.Metzger-Filho O, Moulin C, de Azambuja E, et al. Larotaxel: Broadening the road with new taxanes. Expert Opin Investig Drugs. 2009;18:1183–1189. doi: 10.1517/13543780903119167. [DOI] [PubMed] [Google Scholar]
- 14.Morris PG, Fornier MN. Novel anti-tubulin cytotoxic agents for breast cancer. Expert Rev Anticancer Ther. 2009;9:175–185. doi: 10.1586/14737140.9.2.175. [DOI] [PubMed] [Google Scholar]
- 15.Lee FY, Smykla R, Johnston K, et al. Preclinical efficacy spectrum and pharmacokinetics of ixabepilone. Cancer Chemother Pharmacol. 2009;63:201–212. doi: 10.1007/s00280-008-0727-5. [DOI] [PubMed] [Google Scholar]
- 16.Jimeno A. Eribulin: Rediscovering tubulin as an anticancer target. Clin Cancer Res. 2009;15:3903–3905. doi: 10.1158/1078-0432.CCR-09-1023. [DOI] [PubMed] [Google Scholar]
- 17.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 19.Twelves C, Loesch D, Blum JL, et al. A phase III study (EMBRACE) of eribulin mesylate versus treatment of physician's choice in patients with locally recurrent or metastatic breast cancer previously treated with an anthracycline and a taxane [abstract CRA1004] J Clin Oncol. 2010;28(18 suppl):958s. [Google Scholar]
- 20.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. The Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 21.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 22.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 23.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 24.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 25.Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): Two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 26.Seidman AD, Conlin AK, Bach A, et al. Phase II study of weekly nanoparticle albumin bound (nab)paclitaxel with carboplatin and trastuzumab as 1st-line therapy for HER2-positive metastatic breast cancer (MBC) J Clin Oncol. 2008;26(suppl 15):52s. doi: 10.3816/CBC.2010.n.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moulder S, Li H, Wang M, et al. A phase II trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with HER2-positive metastatic breast cancer: An Eastern Cooperative Oncology Group trial. Breast Cancer Res Treat. 2010;119:663–671. doi: 10.1007/s10549-009-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolaney SM, Najita J, Chen W, et al. A phase II study of ixabepilone plus trastuzumab for metastatic HER2-positive breast cancer. Cancer Res. 2009;69 doi: 10.1093/annonc/mdt121. Abstract 3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 30.GlaxoSmithKline. Research Triangle Park, NC: GlaxoSmithKline; 2010. Tykerb® (lapatinib) tablets [full prescribing information] [Google Scholar]
- 31.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 32.Krop IE, Burris HA, Rugo H, et al. Quantitative assessment of HER2 status and correlation with efficacy for patients (pts) with metastatic breast cancer (MBC) in a phase II study of trastuzumab-DM1 (T-DM1) J Clin Oncol. 2009;27(suppl 15):1003. [Google Scholar]
- 33.Vogel CL, Burris HA, Limentani S, et al. A phase II study of trastuzumab-DM1 (T-DM1), a HER2 antibody-drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC): Final results. J Clin Oncol. 2009;27(suppl 15):1017. [Google Scholar]
- 34.ClinicalTrials.gov. An Open-Label Study of Trastuzumab-MCC-DM1 (T-DM1) vs. Capecitabine + Lapatinib in Patients With HER2-Positive Locally Advanced or Metastatic Breast Cancer (EMILIA) [accessed March 24, 2011]. Available at http://clinicaltrials.gov/show/NCT00829166.
- 35.Gianni L, Lladó A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–1137. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nahta R, Esteva FJ. HER2 therapy: Molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrelli F, Cabiddu M, Cazzaniga ME, et al. Targeted therapies for the treatment of breast cancer in the post-trastuzumab era. The Oncologist. 2008;13:373–381. doi: 10.1634/theoncologist.2007-0173. [DOI] [PubMed] [Google Scholar]
- 39.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer. J Clin Oncol. 2008;26(suppl 15):43s. [Google Scholar]
- 40.Baselga J, Gomez P, Awada A, et al. The addition of cetuximab to cisplatin increases overall response rate (ORR) and progression-free survival (PFS) in metastatic triple-negative breast cancer (TNBC): Results of a randomized phase II study (BALI-1) Ann Oncol. 2010;21(suppl 8):274O. [Google Scholar]
- 41.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 42.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 43.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 44.Blackwell KL, Burstein HJ, Sledge GW, et al. Updated survival analysis of a randomized study of lapatinib alone or in combination with trastuzumab in women with HER2-positive metastatic breast cancer progressing on trastuzumab therapy. Presented at the San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. Abstract 61. [Google Scholar]
- 45.Baselga J, Bradbury I, Eidtmann H, et al. First results of the NeoALTTO trial (BIG 01–06/EGF 106903): A phase III, randomized, open label, neoadjuvant study of lapatinib, trastuzumab, and their combination plus paclitaxel in women with HER2-positive primary breast cancer [abstract 291]. Presented at the San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, Texas. [Google Scholar]
- 46.Tsou HR, Overbeek-Klumpers EG, Hallett WA, et al. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. J Med Chem. 2005;48:1107–1131. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 47.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 48.Miles D, Chan A, Romieu G, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. J Clin Oncol. 2008;26(suppl 15):43s. [Google Scholar]
- 49.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2009;27(suppl 15):1005. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 50.O'Shaughnessy J, Miles D, Gray RJ, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28(suppl 15):1005. [Google Scholar]
- 51.Brufsky A, Rivera RR, Hurvitz SA, et al. Progression-free survival (PFS) in patient subgroups in RIBBON-2, a phase III trial of chemotherapy (chemo) plus or minus bevacizumab (BV) for second-line treatment of HER2-negative, locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2010;28(suppl 15):1021. [Google Scholar]
- 52.Gradishar W, Kaklamani V, Prasad-Sahoo T, et al. A double-blind, randomized, placebo-controlled, phase 2b study evaluating the efficacy and safety of sorafenib (SOR) in combination with paclitaxel (PAC) as first-line therapy in patients (pts) with locally recurrent or metastatic breast cancer (BC) [abstract nr 44] Cancer Res. 2009;69:44. [Google Scholar]
- 53.Baselga J, Segalla JGM, Roché H, et al. SOLTI-0701: A double-blind, randomized phase 2b study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with capecitabine (CAP) in patients with locally advanced (adv) or metastatic (met) breast cancer (BC) Eur J Cancer Suppl. 2009;7:3–4. [Google Scholar]
- 54.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 55.Liljegren A, Bergh J, Castany R. Early experience with sunitinib, combined with docetaxel, in patients with metastatic breast cancer. Breast. 2009;18:259–262. doi: 10.1016/j.breast.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Bergh J, Greil R, Voytko N, et al. Sunitinib (SU) in combination with docetaxel (D) versus D alone for the first-line treatment of advanced breast cancer (ABC) J Clin Oncol. 2010;28(18 suppl):LBA1010. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 57.Crown J, Dieras V, Staroslawska E, et al. Phase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC) J Clin Oncol. 2010;28(18 suppl):LBA1011. [Google Scholar]
- 58.Chew HK, Barlow WE, Albain K, et al. A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: Southwest Oncology Group Study 0338. Clin Breast Cancer. 2008;8:511–515. doi: 10.3816/CBC.2008.n.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: From bench to bedside. Ann Oncol. 2011;22:268–279. doi: 10.1093/annonc/mdq322. [DOI] [PubMed] [Google Scholar]
- 60.Tutt A, Robson M, Garber JE, et al. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer [abstract CRA501] J Clin Oncol. 2009;27(18 suppl):7s. [Google Scholar]
- 61.O'Shaughnessy J, Osborne C, Pippen J, et al. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): Results of a randomized phase II trial [abstract 3] J Clin Oncol. 2009;27(18 suppl):793s. [Google Scholar]
- 62.Gelmon KA, Hirte HW, Robidoux A, et al. Can we define tumors that will respond to PARP inhibitors? A phase II correlative study of olaparib in advanced serous ovarian cancer and triple-negative breast cancer [abstract 3002] J Clin Oncol. 2010;28(15 suppl):233s. [Google Scholar]
- 63.Rodriguez AA, Makris A, Wu MF, et al. DNA repair signature is associated with anthracycline response in triple negative breast cancer patients. Breast Cancer Res Treat. 2010;123:189–196. doi: 10.1007/s10549-010-0983-z. [DOI] [PubMed] [Google Scholar]
- 64.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 65.Cardoso F, Gianni L, Jerusalem G, et al. Multicenter phase I clinical trial of daily and weekly everolimus (RAD001) in combination with vinorelbine and trastuzumab in patients with HER-2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab. Eur J Cancer Suppl. 2009;7:261. [Google Scholar]
- 66.Gui CY, Ngo L, Xu WS, et al. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finnin MS, Donigian JR, Cohen A, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 68.de Ruijter AJ, van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luu TH, Morgan RJ, Leong L, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: A California Cancer Consortium study. Clin Cancer Res. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen S, Ye J, Kijima I, et al. The HDAC inhibitor LBH589 (panobinostat) is an inhibitory modulator of aromatase gene expression. Proc Natl Acad Sci U S A. 2010;107:11032–11037. doi: 10.1073/pnas.1000917107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crevel G, Bates H, Huikeshoven H, et al. The Drosophila Dpit47 protein is a nuclear Hsp90 co-chaperone that interacts with DNA polymerase alpha. J Cell Sci. 2001;114:2015–2025. doi: 10.1242/jcs.114.11.2015. [DOI] [PubMed] [Google Scholar]
- 72.Lee FY, Castaneda S, Inigo I, et al. Ixabepilone (BMS-247550) plus trastuzumab combination chemotherapy induces synergistic antitumor efficacy in HER2 dependent breast cancers and is accompanied by modulation of molecular response markers. J Clin Oncol. 2005;23(16 suppl):19s. [Google Scholar]
- 73.Pegram M, Hsu S, Lewis G, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 74.Pegram MD, Konecny GE, O'Callaghan C, et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 75.Fujimoto-Ouchi K, Sekiguchi F, Tanaka Y. Antitumor activity of combinations of anti-HER-2 antibody trastuzumab and oral fluoropyrimidines capecitabine/5′-dFUrd in human breast cancer models. Cancer Chemother Pharmacol. 2002;49:211–216. doi: 10.1007/s00280-001-0401-7. [DOI] [PubMed] [Google Scholar]